Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 40362799
- PMCID: PMC12073269
- DOI: 10.3390/nu17091490
Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
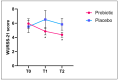
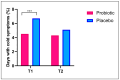
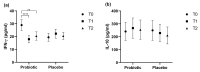
Background: Colds are widespread infectious diseases that affect daily life, increasing healthcare costs and limiting productivity. Objectives: The aim of this study was to investigate the effects of a dietary supplement containing specific probiotic strains (L. plantarum PBS067, L. acidophilus PBS066, B. lactis BL050) on cold symptom relief, immune response enhancement, and quality of life. Methods This randomized, double-blind, placebo-controlled trial included 65 healthy volunteers (age range: 18-44 years), divided into two groups: 40 received the probiotic treatment (with vitamins and bulking agents), and 25 received placebo (vitamins and bulking agents only) for 12 weeks. Cold symptoms and systemic inflammation were assessed at three time points (baseline T0, post-treatment T1, and 6 weeks after treatment T2). Results: Probiotics were associated with a shorter average duration of cold symptoms (4.5 vs. 6.7% for Placebo, p < 0.05). At T1, fever and muscle pain occurred in 20% of participants in the Probiotic group vs. 28% and 44% in the Placebo group, respectively (p < 0.05 for muscle pain vs. Placebo). For muscle pain, a trend was maintained also at T2 (17.5% vs. 20%). The pro-inflammatory cytokine IFN-γ levels significantly decreased in the Probiotic group vs. T0 (p < 0.0001 at T1 and p < 0.01 at T2), while they increased in the Placebo group (22.279 ± 3.538 vs. 19.432 ± 3.143 pg/mL, p = NS). Although not statistically significant, at T1 the Probiotic group had higher levels of IL-10 vs. T0 (266.98 ± 78.432 vs. 240.967 ± 70.238, pg/mL p = NS). Conclusions: The probiotic mix effectively alleviated cold symptoms and reduced pro-inflammatory cytokine levels, suggesting anti-inflammatory effects.
Keywords: URTIs; cold symptoms; food supplements; immunity; probiotics.
Conflict of interest statement
P. Malfa, D.F. Squarzanti, and M. Lo Re are Synbalance srl employees. The sponsor did not participate in the study analysis, construction, or decision about article submission.
Figures




References
-
- Pappas D.E. 26—The Common Cold. In: Long S.S., Prober C.G., Fischer M., editors. Principles and Practice of Pediatric Infectious Diseases. 5th ed. Elsevier; Amsterdam, The Netherlands: 2018. pp. 199–202.e1.
-
- Kobatake E., Iwama Y., Arai T., Shioya N., Kise M., Kabuki T. Intake of Lactobacillus Paragasseri SBT2055 Improves Subjective Symptoms of Common Cold during Winter Season in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Study. Front. Nutr. 2022;9:1063584. doi: 10.3389/fnut.2022.1063584. - DOI - PMC - PubMed
-
- Coleman J.L., Hatch-McChesney A., Small S.D., Allen J.T., Sullo E., Agans R.T., Fagnant H.S., Bukhari A.S., Karl J.P. Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2022;13:2277–2295. doi: 10.1093/advances/nmac086. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

