Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer's Disease, Parkinson's Disease, and Amyotrophic Lateral Sclerosis
- PMID: 40362907
- PMCID: PMC12073124
- DOI: 10.3390/nu17091597
Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer's Disease, Parkinson's Disease, and Amyotrophic Lateral Sclerosis
Abstract
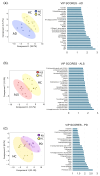
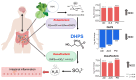
Background: Neurodegenerative diseases (NDDs) are multifactorial disorders frequently associated with gut dysbiosis, oxidative stress, and inflammation; however, the pathophysiological mechanisms remain poorly understood. Methods: Using untargeted mass spectrometry-based metabolomics and 16S sequencing of human stool, we investigated bacterial and metabolic dyshomeostasis in the gut microbiome associated with early disease stages across three NDDs-amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD), Parkinson's disease (PD)-and healthy controls (HC). Results: We discovered a previously unrecognized link between a microbial-derived metabolite with an unknown role in human physiology, 2,3-dihydroxypropane-1-sulfonate (DHPS), and gut dysbiosis in NDDs. DHPS was downregulated in AD, ALS, and PD, while bacteria involved in DHPS metabolism, Eubacterium and Desulfovibrio, were increased in all disease cohorts. Additionally, select taxa within the Clostridia class had strong negative correlations to DHPS, suggesting a potential role in DHPS metabolism. A catabolic product of DHPS is hydrogen sulfide, and when in excess, it is known to promote inflammation, oxidative stress, mitochondrial damage, and gut dysbiosis, known hallmarks of NDDs. Conclusions: These findings suggest that cryptic sulfur metabolism via DHPS is a potential missing link in our current understanding of gut dysbiosis associated with NDD onset and progression. As this was a hypothesis generating study, more work is needed to elucidate the role of DHPS in gut dysbiosis and neurodegenerative diseases.
Keywords: 2,3-dihydroxypropane-1-sulfonate (DHPS); Alzheimer’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; bacterial-derived; metabolomics; microbiome; neurodegenerative disease.
Conflict of interest statement
The authors have no conflicts of interest to disclose. J.C.E. is an employee of NetEllis, LLC.
Figures


References
-
- Feigin V.L., Abajobir A.A., Abate K.H., Abd-Allah F., Abdulle A.M., Abera S.F., Abyu G.Y., Ahmed M.B., Aichour A.N., Aichour I., et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. - DOI - PMC - PubMed
-
- Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. - DOI - PMC - PubMed
-
- Berry J.D., Blanchard M., Bonar K., Drane E., Murton M., Ploug U., Ricchetti-Masterson K., Savic N., Worthington E., Heiman-Patterson T. Epidemiology and economic burden of amyotrophic lateral sclerosis in the United States: A literature review. Amyotroph. Lateral Scler. Front. Degener. 2023;24:436–448. doi: 10.1080/21678421.2023.2165947. - DOI - PubMed
MeSH terms
Grants and funding
- N/A/The Cole Family who support Parkinson's care and research at the Cole Center for Parkinson's Disease and Movement Disorders
- N/A/University of Tennessee at Knoxville's Human Health & Wellness Program
- N/A/Laboratory Directed Research and Development Program at Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the U.S. Department of Energy
- N/A/A philanthropic donor to ALS research at the University of Tennessee Medical Center
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

