Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms
- PMID: 40362912
- PMCID: PMC12073535
- DOI: 10.3390/nu17091602
Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms
Abstract
Background/Objectives: Research on the safety and efficacy of high-dose folinic acid in Chinese children with autism spectrum disorder (ASD) is limited, and the impact of folate metabolism gene polymorphisms on its efficacy remains unclear. This trial aimed to evaluate the safety and efficacy of high-dose folinic acid intervention in Chinese children with ASD and explore the association between folate metabolism gene polymorphisms and efficacy. Methods: A 12-week randomized clinical trial was conducted, including 80 eligible children with ASD, randomly assigned to an intervention group (n = 50) or a control group (n = 30). The intervention group was administered folinic acid (2 mg/kg/day, max 50 mg/day) in two divided doses. Efficacy was measured using the Psycho-Educational Profile, Third Edition (PEP-3) at baseline and 12 weeks by two trained professionals blind to the group assignments. Methylenetetrahydrofolate reductase (MTHFR C677T, MTHFR A1298C), methionine synthase (MTR A2756G), and methionine synthase reductase (MTRR A66G) were genotyped by the gold standard methods in the intervention group. Results: 49 participants in the intervention group and 27 in the control group completed this trial. Both groups showed improvements from baseline to 12 weeks across most outcome measures. The intervention group demonstrated significantly greater improvements in social reciprocity compared to the control group. Children with MTHFR A1298C or MTRR A66G mutations demonstrated greater improvements in various developmental domains than wild type. Folinic acid may be more effective in certain genotype combinations, such as MTHFR C677T and A1298C. No significant adverse effects were observed during the intervention. Conclusions: High-dose folinic acid may be a promising intervention for children with ASD, and its efficacy is associated with folate metabolism gene polymorphisms. High-dose folinic acid intervention may promote better neurodevelopmental outcomes by alleviating folate metabolism abnormalities caused by single or combined mutations in folate metabolism genes.
Keywords: autism; efficacy; folate metabolism gene polymorphisms; folinic acid; safety.
Conflict of interest statement
The authors report no conflicts of interest. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; and the decision to submit the manuscript for publication.
Figures
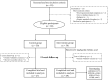
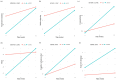
Similar articles
-
Joint associations of folate, homocysteine and MTHFR, MTR and MTRR gene polymorphisms with dyslipidemia in a Chinese hypertensive population: a cross-sectional study.Lipids Health Dis. 2015 Sep 4;14:101. doi: 10.1186/s12944-015-0099-x. Lipids Health Dis. 2015. PMID: 26337056 Free PMC article.
-
Homocysteine Metabolism Gene Polymorphisms (MTHFR C677T, MTHFR A1298C, MTR A2756G and MTRR A66G) Jointly Elevate the Risk of Folate Deficiency.Nutrients. 2015 Aug 10;7(8):6670-87. doi: 10.3390/nu7085303. Nutrients. 2015. PMID: 26266420 Free PMC article. Clinical Trial.
-
[Case-control study on the association between four single nucleotide polymorphisms in folate metabolism way and the risk of congenital heart disease].Wei Sheng Yan Jiu. 2018 Jul;47(4):536-542. Wei Sheng Yan Jiu. 2018. PMID: 30081977 Chinese.
-
MTHFR C677T、MTHFR A1298C、MTRR A66G and MTR A2756G polymorphisms and male infertility risk: a systematic review and meta-analysis.Reprod Biol Endocrinol. 2024 Oct 30;22(1):133. doi: 10.1186/s12958-024-01306-7. Reprod Biol Endocrinol. 2024. PMID: 39478547 Free PMC article.
-
Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review.Am J Epidemiol. 2004 Mar 1;159(5):423-43. doi: 10.1093/aje/kwh066. Am J Epidemiol. 2004. PMID: 14977639 Review.
Cited by
-
The Shuttling of Methyl Groups Between Folate and Choline Pathways.Nutrients. 2025 Jul 30;17(15):2495. doi: 10.3390/nu17152495. Nutrients. 2025. PMID: 40806080 Free PMC article. Review.
References
-
- Dudley C., Emery J.C.H. The Value of Caregiver Time: Costs of Support and Care for Individuals Living with Autism Spectrum Disorder. 2014. [(accessed on 15 January 2014)]. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2379633.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

