Physical Stability and Molecular Mobility of Resveratrol in a Polyvinylpyrrolidone Matrix
- PMID: 40363721
- PMCID: PMC12073277
- DOI: 10.3390/molecules30091909
Physical Stability and Molecular Mobility of Resveratrol in a Polyvinylpyrrolidone Matrix
Abstract
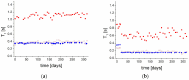
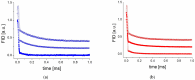
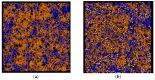
The physical stability, molecular mobility, and appearance of nanocrystalline resveratrol in a polyvinylpyrrolidone (PVP) matrix were investigated. Two formulations with resveratrol loadings of 30% and 50% were prepared and characterized using powder X-ray diffraction (PXRD) and time-domain nuclear magnetic resonance (TD-NMR). Samples were studied over time (up to 300 days post-preparation), across temperatures (80-300 K), and under varying humidity conditions (0% and 75% relative humidity). The results demonstrate that the 30% resveratrol-PVP sample is a homogeneous amorphous solid dispersion (ASD), while the 50% resveratrol-PVP sample contained resveratrol nanocrystals measuring about 40 nm. NMR measurements and molecular dynamics (MD) simulations revealed that incorporation of resveratrol into the polymer matrix modifies the system's dynamics and mobility compared to the pure PVP polymer. Additionally, MD simulations analyzed the hydrogen bonding network within the system, providing insights for a better understanding of the physical stability of the ASD under different conditions.
Keywords: molecular mobility; physical stability; polyvinylpyrrolidone; relaxometry; resveratrol; time-domain NMR.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
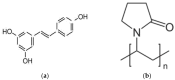






Similar articles
-
The Physical Stability of Felodipine and Its Recrystallization from an Amorphous Solid Dispersion Studied by NMR Relaxometry.AAPS PharmSciTech. 2022 Mar 21;23(4):93. doi: 10.1208/s12249-022-02234-8. AAPS PharmSciTech. 2022. PMID: 35314906
-
Crystallization of amorphous solid dispersions of resveratrol during preparation and storage-Impact of different polymers.J Pharm Sci. 2013 Jan;102(1):171-84. doi: 10.1002/jps.23358. Epub 2012 Nov 6. J Pharm Sci. 2013. PMID: 23132686
-
Variable-Temperature NMR Analysis of the Thermodynamics of Polymer Partitioning between Aqueous and Drug-Rich Phases and Its Significance for Amorphous Formulations.Mol Pharm. 2022 Jan 3;19(1):100-114. doi: 10.1021/acs.molpharmaceut.1c00664. Epub 2021 Oct 26. Mol Pharm. 2022. PMID: 34702040
-
Effect of Drug-Polymer Interaction in Amorphous Solid Dispersion on the Physical Stability and Dissolution of Drugs: The Case of Alpha-Mangostin.Polymers (Basel). 2023 Jul 13;15(14):3034. doi: 10.3390/polym15143034. Polymers (Basel). 2023. PMID: 37514423 Free PMC article.
-
Structural and dynamic properties of amorphous solid dispersions: the role of solid-state nuclear magnetic resonance spectroscopy and relaxometry.J Pharm Sci. 2014 Sep;103(9):2635-2662. doi: 10.1002/jps.23966. Epub 2014 Apr 8. J Pharm Sci. 2014. PMID: 24715618 Review.
References
LinkOut - more resources
Full Text Sources

