Fast-Track Diagnostic Pathway for Lung Cancer Detection: Single-Center Experience
- PMID: 40363946
- PMCID: PMC12072635
- DOI: 10.3390/jcm14092915
Fast-Track Diagnostic Pathway for Lung Cancer Detection: Single-Center Experience
Abstract
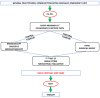
Objectives: Despite continuous advances in diagnosis, such as the "Two week wait" policy for hospital specialist referral and fast-track diagnostic pathways, lung cancers are detected mostly at advanced stages. Our aim was to evaluate the fast-track diagnostic pathway in a tertiary hospital. Methods: Between March and September 2022, 114 consecutive patients with respiratory symptoms or radiology suspicions of lung cancer were referred to our "Pulmonary Point" outpatient clinic. The time intervals to take in the charges and conduct biopsy and 18FDGPET-CT were prospectively collected. Furthermore, the patients' experiences were evaluated by means of a six-item questionnaire investigating the outpatient clinic environment and accessibility, the kindness and professional approach of the healthcare professionals, the psychological support provided and an overall evaluation. The data were compared with those of 79 patients observed in the Thoracic Surgery Ambulatory in the pre-COVID-19 pandemic period of March-September 2019 before the fast-track diagnostic pathway for lung cancer was established. Results: The patients were referred to the "Pulmonary Point" outpatient clinic by a General Practitioner in 44 cases (38.5%), by other Specialists in 56 (49.1%) and by an Emergency Department in 14 (12.2%). Among the 114 patients, 104 (91.2%) were visited within 3 working days. Biopsies (FNAB, EBUS, bronchoscopy or surgical) were performed at a median period of 18 days (IQR: 9-26), and 18FDGPET-CT was carried out at a median period of 16 days (IQR: 7-25). The patients referred to the Thoracic Surgery Ambulatory in the period of March-September 2019 were characterized by longer times to biopsy [26 days (IQR: 12-54), p < 0.001] and to 18FDGPET-CT [25 days (IQR: 15-38), p = 0.003]. The patients referred in 2022 reported higher scores in the clinic environment (p < 0.001), psychological support provided (p < 0.001) and overall evaluation (p = 0.02) domains of the questionnaire. Conclusions: The establishment of a dedicated diagnostic pathway improves time to diagnosis and patients' satisfaction.
Keywords: diagnostic pathways; lung cancer early diagnosis; multidisciplinary team; patients’ satisfaction; time to 18FDGPET-CT; time to biopsy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cantini L., Mentrasti G., Russo G.L., Signorelli D., Pasello G., Rijavec E., Russano M., Antonuzzo L., Rocco D., Giusti R., et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): Fewer cases and higher stages from a real-world scenario. ESMO Open. 2022;7:100406. doi: 10.1016/j.esmoop.2022.100406. Erratum in: ESMO Open 2022, 7, 100471. https://doi.org/10.1016/j.esmoop.2022.100471 . - DOI - PMC - PubMed
-
- Rusch V.W., Parekh K.R., Leon L., Venkatraman E., Bains M.S., Downey R.J., Boland P., Bilsky M., Ginsberg R.J. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J. Thorac. Cardiovasc. Surg. 2000;119:1147–1153. doi: 10.1067/mtc.2000.106089. - DOI - PubMed
-
- Cai J.S., Li Y., Yang F., Wang X. Investigation of the non-size-determined T4N0-2M0 non-small-cell lung cancer: What is the proper T category for the tumour with additional nodules in different lobes of ipsilateral lung? Eur. J. Cardio-Thoracic Surg. 2023;64:ezad243. doi: 10.1093/ejcts/ezad243. - DOI - PubMed
LinkOut - more resources
Full Text Sources

