Planned vs. Performed Treatment Regimens in Diabetic Macular Edema: Real-World Evidence from the PACIFIC Study
- PMID: 40364151
- PMCID: PMC12072375
- DOI: 10.3390/jcm14093120
Planned vs. Performed Treatment Regimens in Diabetic Macular Edema: Real-World Evidence from the PACIFIC Study
Abstract
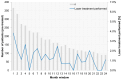
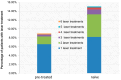
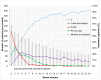
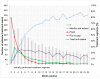
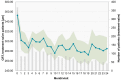
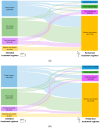
Background: Intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors are standard for diabetic macular edema (DME), yet a gap exists between clinical guidelines and actual practices. This study aimed to investigate the extent of deviation between physician-planned and actually performed treatment regimens. Methods: The PACIFIC study (NCT04847895) was a prospective, multicenter, non-interventional study conducted in Germany, the Netherlands, and Switzerland. A total of 910 patients with DME receiving ranibizumab were enrolled. Physicians documented the intended treatment regimen at baseline, and actual treatment patterns were retrospectively derived from the timing of visits and injections over a 24-month observation period. Results: Although most physicians initially planned fixed or pro re nata (PRN) regimens, 77% of pretreated and 73% of treatment-naïve patients ultimately followed a monitor and extend strategy. Treatment discontinuation was frequent (58.8% and 59.4%, respectively), and injection frequencies remained below recommended levels, although central retinal thickness improved over time. Conclusions: The study highlights a consistent and clinically relevant discrepancy between planned and actual treatment delivery in DME care, underscoring the need for better adherence to guideline-informed strategies in routine practice.
Keywords: clinical guidelines; diabetic macular edema (DME); intravitreal injections; observational study; patient-centered care; real-world evidence; treatment adherence; treatment deviations; treatment strategies; vascular endothelial growth factor (VEGF) inhibitors.
Conflict of interest statement
Christos Haritoglou received honoraria as a speaker from Novartis, Bayer, and Allergan/AbbVie. Matthias Iwersen and Bettina Müller are employees of Novartis Pharma GmbH, Germany. Erik Beeke received a grant from Novartis. Hüsnü Berk declares no conflicts of interest outside the study participation fees. Matthias Grüb received grants from Novartis and Bayer and personal fees from Novartis. Katrin Lorenz received honoraria from Ethikkommission der Landesärztekammer Rheinland-Pfalz and Novartis Pharma GmbH, travel grants from Novartis Pharma GmbH, and participated in the following clinical trials/grants: Aerie, Allergan, Amgen, Bayer, Chengdu Kanghong Biotechnology Co., Hexal, Hoya, iStar, Iveric Bio, Janssen Cilag, Implandata, Lumithera, Microoptx, Mylan, Novartis, Ophtea limited, Pfizer, Redwood, Roche, Sensimed, and Santen. Martin Scheffler declares no conflicts of interest outside the study participation fees. Focke Ziemssen received grants or personal fees from Acelyrin, Alimera, Allergan/Abbvie, Apellis, Bayer Healthcare, BDI, Biogen, Boehringer-Ingelheim, Clearside, CME Health, Ionis, Janssen, Kodiak, Novartis, NovoNordisk, MSD Sharp & Dohme, Oxurion, ODOS, Ophtea, Regeneron, Roche/Genentech, Sandoz, Sanofi, and Stada.
Figures



References
-
- Comyn O., Sivaprasad S., Peto T., Neveu M.M., Holder G.E., Xing W., Bunce C.V., Patel P.J., Egan C.A., Bainbridge J.W., et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study) Am. J. Ophthalmol. 2014;157:960–970. doi: 10.1016/j.ajo.2014.02.019. - DOI - PubMed
-
- Massin P., Bandello F., Garweg J.G., Hansen L.L., Harding S.P., Larsen M., Mitchell P., Sharp D., Wolf-Schnurrbusch U.E.K., Gekkieva M., et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. - DOI - PMC - PubMed
-
- Nguyen Q.D., Brown D.M., Marcus D.M., Boyer D.S., Patel S., Feiner L., Gibson A., Sy J., Rundle A.C., Hopkins J.J., et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. - DOI - PubMed
-
- Ishibashi T., Li X., Koh A., Lai T.Y., Lee F.L., Lee W.K., Ma Z., Ohji M., Tan N., Cha S.B., et al. The REVEAL Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy in Asian Patients with Diabetic Macular Edema. Ophthalmology. 2015;122:1402–1415. doi: 10.1016/j.ophtha.2015.02.006. - DOI - PubMed
-
- Kodjikian L., Lecleire-Collet A., Dot C., Le Lez M., Baillif S., Erginay A., Souied E., Fourmaux E., Gain P., Ponthieux A. ETOILE: Real-World Evidence of 24 Months of Ranibizumab 0.5 mg in Patients with Visual Impairment Due to Diabetic Macular Edema. Clin. Ophthalmol. 2021;15:2307–2315. doi: 10.2147/OPTH.S313081. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

