The Supporting Role of Hyperbaric Oxygen Therapy in Atopic Dermatitis Treatment
- PMID: 40364168
- PMCID: PMC12072933
- DOI: 10.3390/jcm14093138
The Supporting Role of Hyperbaric Oxygen Therapy in Atopic Dermatitis Treatment
Abstract
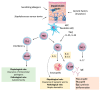
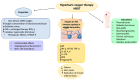
Over the past decades, atopic diseases have emerged as a growing global health concern. The Global Report on Atopic Dermatitis 2022 estimated that approximately 223 million people worldwide were living with atopic dermatitis in 2022, with around 43 million being children or adolescents. The financial burden associated with the treatment of this condition poses a significant challenge for both healthcare systems and patients. The current therapeutic approach for atopic diseases primarily focuses on symptomatic management, aiming to mitigate the effects of an overactive immune system. The most widely used treatments include topical or systemic corticosteroids, which suppress inflammation, and emollients, which help restore the skin barrier function. However, prolonged corticosteroid use is associated with adverse effects, including impaired immune response and reduced ability to combat external and internal threats. Consequently, there is a growing interest in developing alternative therapeutic strategies for managing atopic dermatitis. Among these emerging treatments, hyperbaric oxygen therapy (HBOT) appears particularly promising. HBOT has a beneficial effect on the vascular and immune systems, which results in improved functioning of tissues and organs. This therapy has demonstrated efficacy in promoting wound healing, particularly in conditions such as thermal burns and diabetic foot ulcers. Given these properties, HBOT is being tested as a potential adjunctive therapy for atopic dermatitis and other allergy-related diseases. In this paper, we present the current state of knowledge regarding the application of HBOT in the treatment of atopic and immune-mediated conditions, with a focus on its immunomodulatory and regenerative effects.
Keywords: HBOT; atopic dermatitis; immune system; immunomodulation; supporting therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Asher M.I., Montefort S., Björkstén B., Lai C.K.W., Strachan D.P., Weiland S.K., Williams H. ISAAC Phase Three Study Group Worldwide Time Trends in the Prevalence of Symptoms of Asthma, Allergic Rhinoconjunctivitis, and Eczema in Childhood: ISAAC Phases One and Three Repeat Multicountry Cross-Sectional Surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

