Erythroid-stimulating agents in VEXAS syndrome: A retrospective study from an Italian multicentre cohort
- PMID: 40364459
- PMCID: PMC12234262
- DOI: 10.1111/bjh.20157
Erythroid-stimulating agents in VEXAS syndrome: A retrospective study from an Italian multicentre cohort
Keywords: VEXAS; erythropoietin; macrocytic anaemia; myelodysplastic neoplasms.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
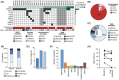
References
-
- Georgin‐Lavialle S, Terrier B, Guedon AF, Heiblig M, Comont T, Lazaro E, et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large‐scale analysis of a multicentre case series of 116 French patients. Br J Dermatol. 2021;186(3):564–574. 10.1111/bjd.20805 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

