Human and Mouse Alzheimer's Seeds Differentially Affect Amyloid Deposition and Microglia-Dependent Plaque Response in Aged Mice
- PMID: 40364523
- PMCID: PMC12341799
- DOI: 10.1111/acel.70094
Human and Mouse Alzheimer's Seeds Differentially Affect Amyloid Deposition and Microglia-Dependent Plaque Response in Aged Mice
Abstract
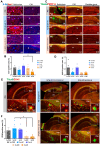
Alzheimer's disease (AD) is a complex neurodegenerative proteinopathy in which Aβ and tau misfold and aggregate into entities that structurally unsettle native proteins, mimicking a prion-like or "seeding" process. These Aβ and tau "seeds" can arrange in different conformations or strains that might display distinct pathogenic properties. Furthermore, recent evidence suggests that microglia play a key role in the amyloidogenic event and can modulate the propagation and aggregation processes. Here, we employed histological and molecular approaches to determine whether seeds from human AD brains compared to those from transgenic mice (3xTg-AD) are more prone to induce Aβ and tau aggregates in vivo, as well as potential differences in the microglial response to the plaque pathology. Brain homogenates were injected into the hippocampus of 3xTg-AD mice and hAβ-KI mice and examined at 18-20 months of age. The seeds from the human AD brain induced more aggressive amyloid pathology compared to seeds from aged 3xTg-AD mice. However, the AD seeds from aged transgenic mice triggered more tau pathology. Interestingly, such mice seeds impaired microglial clustering around plaques, leading to more severe neuritic pathology. Furthermore, the human AD seeds injected into the hippocampus of hAβ-KI mice were not able to induce plaque formation. These results suggest that multiple variables such as the AD seed, recipient model, and time are critical factors that can modulate the amyloid pathology onset and progression. Thus, more profound understanding of these factors will provide key insight into how amyloid and tau pathology progresses in AD.
Keywords: Alzheimer's disease; amyloid‐beta; inflammation; propagation; seeds; tau; transgenic mice.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

