Impact of Aspirin on Timing of Birth in Pregnancies With Clinical Manifestations of Placental Dysfunction: Evidence From a Multicentre Randomised Clinical Trial
- PMID: 40364747
- PMCID: PMC12315083
- DOI: 10.1111/1471-0528.18211
Impact of Aspirin on Timing of Birth in Pregnancies With Clinical Manifestations of Placental Dysfunction: Evidence From a Multicentre Randomised Clinical Trial
Abstract
Objective: To examine whether aspirin delays gestational age at delivery (GAD) in pregnancies with placental dysfunction (PD) phenotypes (preeclampsia [PE], small-for-gestational-age [SGA], placental abruption and/or stillbirth).
Design: A secondary analysis of a multicentre stepped-wedge cluster randomised trial.
Setting: 18 maternity/diagnostic units in Asia.
Population: Singleton pregnancies examined at 11-13+6 weeks.
Methods: A model in which the effect of aspirin is to delay the GAD in pregnancies with PD was developed.
Main outcome measures: GAD in pregnancies with PD.
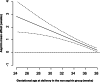
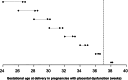
Results: Aspirin administration was associated with a significant reduction in PD < 32 weeks (adjusted relative risk 0.543, 95% CI: 0.330-0.864), with a trend for an increase of PD ≥ 32 weeks (test for trend, p-value = 0.0018). Similar findings were observed individually for PE, SGA and/or placental abruption. At 24 weeks, the aspirin-induced prolongation of pregnancies with PD was 2.85 weeks (95% CI: 0.44-5.40), and this effect was decreased by -0.19 weeks (95% CI: -0.33 to -0.05) for each week of gestation; therefore, at 28 and 32 weeks' gestation, the aspirin-induced prolongation was 2.09 and 1.33 weeks, respectively.
Conclusions: In this secondary analysis of a cluster randomised trial, women at high risk of PE who are destined to develop a clinical spectrum of PD may benefit from longer pregnancy duration through aspirin administration in early pregnancy. Aspirin may delay the GAD due to PD, particularly benefiting those deliveries that would occur at earlier gestations without aspirin administration.
Keywords: aspirin; placental dysfunction; pre‐eclampsia.
© 2025 The Author(s). BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
L.C. Poon has received speaker fees and consultancy payments from Roche Diagnostics, Ferring Pharmaceuticals, Shenzhen Mindray Bio‐Medical Electronics Co. Ltd. and Samsung Healthcare. In addition, she serves as the CEO of PregnaSense Co. Limited and has received in‐kind contributions from Roche Diagnostics, Revvity Inc. (formerly PerkinElmer Life and Analytical Sciences), ThermoFisher Scientific, Ningbo Aucheer Biological Technology Co. Ltd., Samsung Healthcare and GE Healthcare. D.S. Sahota has received in‐kind contributions from Revvity Inc. (formerly PerkinElmer Life and Analytical Sciences), Thermo Fisher Scientific, Roche Diagnostics, Diabetomics and Ningbo Aucheer Biological Technology Co. Ltd. R.K. Pooh is the CEO of Ritz Medical Co. Ltd., a genetic testing company, holds shares in it, and receives executive compensation from it. Other authors declare no conflicts of interest.
Figures
References
-
- Ananth C. V., “Ischemic Placental Disease: A Unifying Concept for Preeclampsia, Intrauterine Growth Restriction, and Placental Abruption,” Seminars in Perinatology 38, no. 3 (2014): 131–132. - PubMed
-
- Morris R. K., Johnstone E., Lees C., Morton V., Smith G., and Royal College of Obstetricians and Gynaecologists , “Investigation and Care of a Small‐For‐Gestational‐Age Fetus and a Growth Restricted Fetus (Green‐Top Guideline No. 31),” BJOG 131, no. 9 (2024): e31–e80. - PubMed
-
- von Dadelszen P., Syngelaki A., Akolekar R., Magee L. A., and Nicolaides K. H., “Preterm and Term Pre‐Eclampsia: Relative Burdens of Maternal and Perinatal Complications,” BJOG: An International Journal of Obstetrics and Gynaecology 130, no. 5 (2023): 524–530, 10.1111/1471-0528.17370. - DOI - PubMed
-
- Papastefanou I., Ashoor G., Syngelaki A., Akolekar R., and Nicolaides K. H., “Relation of Antepartum Stillbirth to Birthweight and Gestational Age: Prospective Cohort Study,” BJOG: An International Journal of Obstetrics and Gynaecology 131, no. 2 (2024): 200–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

