Characterizing spatial immune architecture in metastatic melanoma using high-dimensional multiplex imaging
- PMID: 40364843
- PMCID: PMC12069457
- DOI: 10.3389/fimmu.2025.1560778
Characterizing spatial immune architecture in metastatic melanoma using high-dimensional multiplex imaging
Abstract
Introduction: Immune checkpoint inhibitors (ICIs) have significantly improved survival for patients with metastatic melanoma, yet many experienceresistance due to immunosuppressive mechanisms within the tumor immune microenvironment (TIME). Understanding how the spatial architecture of immune and inflammatory components changes across disease stages may reveal novel prognostic biomarkers and therapeutic targets.
Methods: We performed high-dimensional spatial profiling of two melanoma tissue microarrays (TMAs), representing Stage III (n = 157) and Stage IV (n = 248) metastatic tumors. Using imaging mass cytometry (IMC) and multiplex immunofluorescence (mIF), we characterized the phenotypic, functional, and spatial properties of the TIME. Cellular neighborhoods were defined by inflammatory marker expression, and spatial interactions between immune and tumor cells were quantified using nearest-neighbor functions (G-cross). Associations with survival were assessed using Cox proportional hazards models with robust variance estimation.
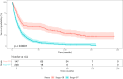
Results: Stage IV tumors exhibited a distinct immune landscape, with increased CD74- and MIF-enriched inflammatory neighborhoods and reduced iNOS-associated regions compared to Stage III. Cytotoxic T lymphocytes (CTLs) and tumor cells were more prevalent in Stage IV TIME, while B cells and NK cells were depleted. Spatial analysis revealed that CTL-Th cell, NK-T cell, and B-NK cell interactions were linked to improved survival, whereas macrophage aggregation and excessive B-Th cell clustering in inflammatory regions correlated with worse outcomes. Organ-specific analyses showed that CTL infiltration near tumor cells predicted survival in gastrointestinal metastases, while NK-T cell interactions were prognostic in lymph node and skin metastases.
Discussion: Our results reveal stage-specific shifts in immune composition and spatial organization within the melanoma TIME. In advanced disease, immunosuppressive neighborhoods emerge alongside changes in immune cell localization, with spatial patterns of immune coordination-particularly involving CTLs, NK cells, and B cells-strongly predicting survival. These findings highlight spatial biomarkers that may refine patient stratification and guide combination immunotherapy strategies targeting the inflammatory architecture of the TIME.
Keywords: immune cell crosstalk; immune exclusion; inflammatory biomarkers; inflammatory signaling pathways; melanoma progression; prognostic immune signatures; spatial immune profiling; tumor immune microenvironment (TIME).
Copyright © 2025 Eliason, Krishnan, Fukuda, Bustos, Winkowski, Cho, Basi, Baird, Grimm, Davies, Hoon, Rao, Burks and Ekmekcioglu.
Conflict of interest statement
AR serves as a member for Voxel Analytics LLC and consults for Genophyll LLC, Tempus Inc. and TCS Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures










References
-
- American Cancer Society . Cancer Facts and Figures 2024. Atlanta: American Cancer Society; (2024).
-
- American Cancer Society . Cancer Facts and Figures 2021. Atlanta: American Cancer Society; (2021).
-
- American Cancer Society . Cancer Facts and Figures 2022. Atlanta: American Cancer Society; (2022).
-
- Chang C-Y, Park H, Malone DC, Wang C-Y, Wilson DL, Yeh Y-M, et al. . Immune checkpoint inhibitors and immune-related adverse events in patients with advanced melanoma: A systematic review and network meta-analysis. JAMA Netw Open. (2020) 3:e201611. doi: 10.1001/jamanetworkopen.2020.1611 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

