Distorted 2H/1T MoS2 nanostructures with improved field emission and sodium-ion battery performance
- PMID: 40364972
- PMCID: PMC12067292
- DOI: 10.1039/d5na00004a
Distorted 2H/1T MoS2 nanostructures with improved field emission and sodium-ion battery performance
Abstract
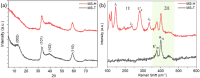
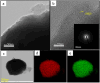
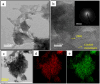
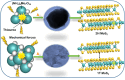
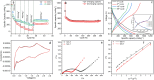
The present investigation demonstrates a facile and scalable synthesis strategy for the preparation of 2H and 1T phases of MoS2 using a solid-state method. Our findings reveal that MoS2 exhibits a distorted octahedral coordination structure, formed owing to the thiourea concentration. The structural transitions from the semiconducting 2H phase to the metallic 1T phase have been confirmed by XRD and Raman spectroscopy. The resulting MoS2 (1T) can provide more active sites by forming an ultrathin monolayer with a thickness of a few nanometers, enhancing the electrical conductivity. Field electron emission (FEE) behavior was investigated at a base pressure of 1 × 10-8 mbar. The utmost emission current density i.e. 820 μA cm-2 was observed for the 1T MoS2 emitter at an applied field of 4.56 V μm-1, in comparison to 2H MoS2, which showed 542 μA cm-2 at 4.85 V μm-1. Considering the good electrical conductivity and layered ultrathin sheet structure, 1T MoS2 has been explored as an anode for SIBs. Interestingly, it delivered a reversible capacity of 870 mA h g-1 at 100 mA g-1, and good cycling performance at 200 mA g-1 with 81% retention after 300 cycles. By integrating MoS2 electrodes with glyme electrolytes, a synergistic effect is achieved, leading to stable electrochemical performance of SIBs. The existence of an active 1T-MoS2 basal plane compared to 2H-MoS2 is responsible for the noticeable field electron emission and SIB application. It is noteworthy that MoS2 without any modifications (such as doping, carbon integration or composite formation) showed excellent performance in both applications. This paper underscores MoS2's versatility and promising avenues for revolutionizing electronic devices and energy storage technologies.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Kadam S. R. Late D. J. Panmand R. P. Kulkarni M. V. Nikam L. K. Gosavi S. W. Park C. J. Kale B. B. J. Mater. Chem. A. 2015;3:21233–21243.
-
- Yin L. Zhang Z. Li Z. Hao F. Li Q. Wang C. Fan R. Qi Y. Adv. Funct. Mater. 2014;24:4176–4185.
-
- Wu J. Liu J. Cui J. Yao S. Ihsan-Ul-Haq M. Mubarak N. Quattrocchi E. Ciucci F. Kim J.-K. J. Mater. Chem. A. 2020;8:2114–2122. doi: 10.1039/C9TA11913B. - DOI
LinkOut - more resources
Full Text Sources

