Scalability of spheroid-derived small extracellular vesicles production in stirred systems
- PMID: 40365014
- PMCID: PMC12069995
- DOI: 10.3389/fbioe.2025.1516482
Scalability of spheroid-derived small extracellular vesicles production in stirred systems
Abstract
Introduction: Small extracellular vesicle (sEV)-based therapies have gained widespread interest, but challenges persist to ensure standardization and high-scale production. Implementing upstream processes in a chemically defined media in stirred-tank bioreactors (STBr) is mandatory to closely control the cell environment, and to scale-up production, but it remains a significant challenge for anchorage-dependent cells.
Methods: We used a human β cell line, grown as monolayer or in suspension as spheroid in stirred systems. We assessed the consequences of culturing these cells in 3D with, or without fetal bovine serum in a chemically defined medium, for cell growth, viability and metabolism. We next explored how different scale-up strategies might influence cell and spheroid formation in spinner flask, with the aim to transfer the process in instrumented Ambr®250 STBr. Lastly, we analyzed and characterized sEV production in monolayer, spinner flask and STBr.
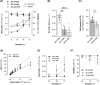
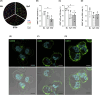
Results and discussion: Generation of spheroids in a chemically defined medium allowed the culture of highly viable cells in suspension in stirred systems. Spheroid size depended on the system's volumetric power input (P/V), and maintaining this parameter constant during scale-up proved to be the optimal strategy for standardizing the process. However, transferring the spinner flask (SpF) process to the Ambr®250 STBr at constant P/V modified spheroid size, due to important geometric differences and impeller design. Compared to a monolayer reference process, sEV yield decreased two-fold in SpF, but increased two-fold in STBr. Additionally, a lower expression of the CD63 tetraspanin was observed in sEV produced in both stirred systems, suggesting a reduced release of exosomes compared to ectosomes. This study addresses the main issues encountered in spheroid culture scale-up in stirred systems, rather conducive for the production of ectosomes.
Keywords: bioprocess; bioproduction; bioreactor; extracellular vesicles; scale-up; shear stress; spheroid.
Copyright © 2025 Dauphin, de Beaurepaire, Salama, Pruvost, Claire, Haurogné, Sourice, Dupont, Bach, Hervé, Olmos, Bosch, Lieubeau and Mosser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Barekzai J., Friedrich J., Okpara M., Refflinghaus L., Eckhardt D., Czermak P., et al. (2023). Dynamic expansion of mesenchymal stem/stromal cells in a stirred tank bioreactor promotes the release of potent extracellular vesicles. AIMS Bioeng. 10, 240–264. 10.3934/bioeng.2023016 - DOI
-
- Berger A., Araújo-Filho I., Piffoux M., Nicolás-Boluda A., Grangier A., Boucenna I., et al. (2021). Local administration of stem cell-derived extracellular vesicles in a thermoresponsive hydrogel promotes a pro-healing effect in a rat model of colo-cutaneous post-surgical fistula. Nanoscale 13, 218–232. 10.1039/D0NR07349K - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

