Hyaluronic acid-loaded drug-eluting nanofibrous pad for the treatment of degenerative arthritis
- PMID: 40365220
- PMCID: PMC12067404
- DOI: 10.1039/d5ra01494h
Hyaluronic acid-loaded drug-eluting nanofibrous pad for the treatment of degenerative arthritis
Abstract
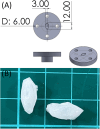
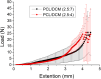
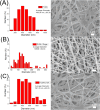
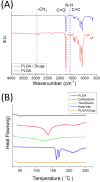
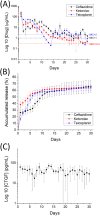
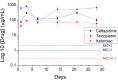
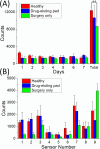
Despite advancements in modern technology, treating degenerative arthritis remains a challenge. This study developed a degradable, hyaluronic acid-loaded, drug-eluting nanofibrous pad designed to provide extended pain relief and prevent infection at the knee joint. The mechanical performance of the biodegradable pads was assessed, and the pharmaceutical discharge kinetics were estimated using an in vitro elution method. Additionally, in vivo pharmaceutical release and efficacy were tested using a rabbit activity model. The experimental results suggest that the degradable pad exhibited strong mechanical properties. In vitro, the drug-eluting nanofibrous pad sustained the release of teicoplanin, ceftazidime, and ketorolac for 10, 24, and 30 days, respectively, and maintained high levels of connective tissue growth factor elution over a 30-day period. Moreover, animal testing demonstrated that the pad released significant amounts of antimicrobial and pain-relieving agents in a rabbit knee joint model for over 28 days. Rabbits implanted with the drug-eluting pads exhibited activity levels comparable to those that did not undergo surgery. These findings indicate that the hyaluronic acid-loaded, drug-eluting nanofibrous degradable pad, with its extended release of pharmaceuticals and biomolecules, may be used for the treatment of degenerative arthritis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors state that there are no conflicts of interest.
Figures



Similar articles
-
Development of novel hybrid 3D-printed degradable artificial joints incorporating electrospun pharmaceutical- and growth factor-loaded nanofibers for small joint reconstruction.Biomater Adv. 2024 May;159:213821. doi: 10.1016/j.bioadv.2024.213821. Epub 2024 Feb 28. Biomater Adv. 2024. PMID: 38428121
-
Lidocaine/ketorolac-loaded biodegradable nanofibrous anti-adhesive membranes that offer sustained pain relief for surgical wounds.Int J Nanomedicine. 2017 Aug 16;12:5893-5901. doi: 10.2147/IJN.S140825. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28860755 Free PMC article.
-
Pharmaceutical-eluting hybrid degradable hydrogel/microparticle loaded sacs for finger joint interpositional arthroplasty.Biomater Adv. 2022 Jun;137:212846. doi: 10.1016/j.bioadv.2022.212846. Epub 2022 May 8. Biomater Adv. 2022. PMID: 35929275
-
Extended pain relief achieved by analgesic-eluting biodegradable nanofibers in the Nuss procedure: in vitro and in vivo studies.Int J Nanomedicine. 2018 Dec 7;13:8355-8364. doi: 10.2147/IJN.S189505. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30573957 Free PMC article.
-
Local cooling for relieving pain from perineal trauma sustained during childbirth.Cochrane Database Syst Rev. 2020 Oct 9;10(10):CD006304. doi: 10.1002/14651858.CD006304.pub4. Cochrane Database Syst Rev. 2020. PMID: 33034900 Free PMC article.
References
-
- Latourte A. Kloppenburg M. Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020;16:673–688. - PubMed
LinkOut - more resources
Full Text Sources

