Efficacy and safety of rituximab for membranous nephropathy in adults: a meta-analysis of RCT
- PMID: 40365242
- PMCID: PMC12069390
- DOI: 10.3389/fneph.2025.1548679
Efficacy and safety of rituximab for membranous nephropathy in adults: a meta-analysis of RCT
Abstract
Background: Membranous nephropathy (MGN) represents a significant challenge in nephrology, with Rituximab emerging as a potential therapeutic intervention.
Methods: A comprehensive systematic review was conducted using PubMed, EMBASE, and Web of Science databases, focusing exclusively on randomized controlled trials (RCTs) from January 2002 to November 2024. Stringent eligibility criteria were applied, including studies with at least ten participants, with data extracted by two independent reviewers. The meta-analysis utilized fixed and random effects models to assess Rituximab's efficacy and safety across multiple outcome measures.
Results: The meta-analysis revealed nuanced findings across different follow-up periods. At 6 months, complete remission rates showed non-significant odds ratios ranging from 2.12 to 2.48. By 12 months, the pooled odds ratio was 0.8085 (95% CI: 0.2238-2.9213), with complete remission rates varying between 13.8% and 19.4%. Notably, at 24 months, the common effects model demonstrated a statistically significant odds ratio of 5.0792 (95% CI: 2.2609-11.4107, p < 0.0001). Proteinuria reduction showed consistent improvement, with a median difference of 4.3225. Adverse event analysis indicated a relatively low risk, with an odds ratio of 0.9706 (95% CI: 0.5781-1.6297).
Conclusion: Rituximab demonstrates potential efficacy in treating MGN, with promising long-term outcomes and a favorable adverse event profile.
Keywords: RCT; membranous nephropathy; meta-analysis; proteinuria; rituximab.
Copyright © 2025 Mao, Han, Wang and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





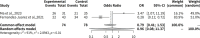

Similar articles
-
Clinical efficacy and safety of rituximab with membranous nephropathy: a meta-analysis.Arch Med Sci. 2020 Oct 14;19(2):411-419. doi: 10.5114/aoms.2020.99899. eCollection 2023. Arch Med Sci. 2020. PMID: 37034519 Free PMC article. Review.
-
Efficacy and safety of rituximab in the treatment of membranous nephropathy: A systematic review and meta-analysis.Medicine (Baltimore). 2020 Apr;99(16):e19804. doi: 10.1097/MD.0000000000019804. Medicine (Baltimore). 2020. PMID: 32311997 Free PMC article.
-
Efficacy and safety of rituximab therapy for membranous nephropathy: a meta-analysis.Eur Rev Med Pharmacol Sci. 2018 Nov;22(22):8021-8029. doi: 10.26355/eurrev_201811_16431. Eur Rev Med Pharmacol Sci. 2018. PMID: 30536351
-
Evaluation of efficacy of rituximab for membranous nephropathy: A systematic review and meta-analysis of 11 studies.Nephrol Ther. 2022 Apr;18(2):104-112. doi: 10.1016/j.nephro.2021.10.002. Epub 2022 Jan 21. Nephrol Ther. 2022. PMID: 35074299
-
Rituximab or Cyclophosphamide in the Treatment of Membranous Nephropathy: The RI-CYCLO Randomized Trial.J Am Soc Nephrol. 2021 Apr;32(4):972-982. doi: 10.1681/ASN.2020071091. Epub 2021 Mar 1. J Am Soc Nephrol. 2021. PMID: 33649098 Free PMC article.
References
LinkOut - more resources
Full Text Sources

