Fish-derived biomaterials for tissue engineering: advances in scaffold fabrication and applications in regenerative medicine and cancer therapy
- PMID: 40365274
- PMCID: PMC12068294
- DOI: 10.7150/thno.109186
Fish-derived biomaterials for tissue engineering: advances in scaffold fabrication and applications in regenerative medicine and cancer therapy
Abstract
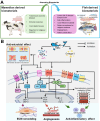
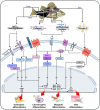
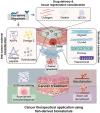
Fish-derived biomaterials, such as collagen, polyunsaturated fatty acids, and antimicrobial peptides, have emerged as promising candidates for scaffold development in stem cell therapies and tissue engineering due to their excellent biocompatibility and low immunogenicity. Although good bioactivity is a prerequisite for biomedical substitutes, scaffold design is necessary for the successful development of bioconstructs used in tissue regeneration. However, the limited processability of fish biomaterials poses a substantial challenge to the development of diverse scaffold structures. In this review, unlike previous reviews that primarily focused on the bioactivities of fish-derived components, we placed greater emphasis on scaffold fabrication and its applications in tissue regeneration. Specifically, we examined various cross-linking strategies to enhance the structural integrity of fish biomaterials and address challenges, such as poor processability, low mechanical strength, and rapid degradation. Furthermore, we demonstrated the potential of fish scaffolds in stem cell therapies, particularly their capacity to support stem cell growth and modulate the cellular microenvironment. Finally, this review provides future directions for the application of these scaffolds in cancer therapy.
Keywords: cancer therapeutics; fish-derived biomaterials; scaffold structure; stem cell activation; tissue engineering.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
The Combination of Bioactive Herbal Compounds with Biomaterials for Regenerative Medicine.Tissue Eng Part B Rev. 2024 Dec;30(6):607-630. doi: 10.1089/ten.TEB.2024.0002. Epub 2024 Apr 12. Tissue Eng Part B Rev. 2024. PMID: 38481114 Review.
-
The Potential Application of Biomaterials in Cardiac Stem Cell Therapy.Curr Med Chem. 2016;23(6):589-602. doi: 10.2174/092986732306160303151041. Curr Med Chem. 2016. PMID: 26951086 Review.
-
Nonwoven membranes for tissue engineering: an overview of cartilage, epithelium, and bone regeneration.J Biomater Sci Polym Ed. 2019 Aug;30(12):1026-1049. doi: 10.1080/09205063.2019.1620592. Epub 2019 Jun 2. J Biomater Sci Polym Ed. 2019. PMID: 31106705
-
Stem Cell-Laden Engineered Patch: Advances and Applications in Tissue Regeneration.ACS Appl Bio Mater. 2025 Jan 20;8(1):62-87. doi: 10.1021/acsabm.4c01427. Epub 2024 Dec 19. ACS Appl Bio Mater. 2025. PMID: 39701826 Review.
-
The Application of Biomaterial-Based Spinal Cord Tissue Engineering.Macromol Biosci. 2025 Mar;25(3):e2400444. doi: 10.1002/mabi.202400444. Epub 2024 Oct 29. Macromol Biosci. 2025. PMID: 39472074 Review.
References
-
- Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. - PubMed
-
- Qin D, Bi S, You X, Wang M, Cong X, Yuan C. et al. Development and application of fish scale wastes as versatile natural biomaterials. Chem Eng J. 2022;428:131102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

