pH-responsive nano-vaccine combined with anti-PD-1 antibodies for enhanced immunotherapy of breast cancer
- PMID: 40365283
- PMCID: PMC12068292
- DOI: 10.7150/thno.107200
pH-responsive nano-vaccine combined with anti-PD-1 antibodies for enhanced immunotherapy of breast cancer
Abstract
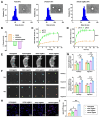
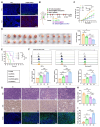
Objective: This study aimed to investigate the therapeutic potential and underlying mechanisms of a novel pH-responsive nano-vaccine in combination with anti-Programmed Cell Death Protein 1 (PD-1) antibodies for the treatment of breast cancer (BC), with a focus on tumor growth inhibition, metastasis prevention, and immune microenvironment modulation. Methods: A pH-responsive amphiphilic diblock copolymer was synthesized using reversible addition-fragmentation chain transfer (RAFT) polymerization and conjugated with STING agonist ADU-S100 and mannose to specifically target dendritic cells (DCs). The nano-vaccine was further formulated with antigen peptides and polyethyleneimine (PEI) to enhance antigen delivery. Its particle size, stability, and surface charge were characterized using dynamic light scattering (DLS) and zeta potential analysis. In vitro, the immunostimulatory capacity of the nano-vaccine was evaluated via flow cytometry (FCM) analysis of DC activation markers. In vivo, mouse immune and tumor recurrence models were used to assess the its effects on T-cell activation, tumor suppression, and immune memory induction. The therapeutic efficacy of nano-vaccine/anti-PD-1 combination therapy was further assessed. Results: The nano-vaccine efficiently activated DCs and promoted antigen presentation, as indicated by increased CD80, CD86, and MHC-II expression in vitro. In mouse models, it effectively inhibited tumor growth, induced antigen-specific T-cell responses, and suppressed recurrent and metastatic tumor progression. The combination with anti-PD-1 antibodies further enhanced tumor control, immune cell infiltration, and survival rates compared to monotherapy. Conclusion: The pH-responsive nano-vaccine combined with anti-PD-1 antibodies showed remarkable synergistic effects in BC treatment, highlighting its potential to enhance immune checkpoint blockade therapy and offer a promising strategy for clinical applications in solid tumors.
Keywords: antigen delivery; breast cancer; immune memory; immunotherapy; nano-vaccine; programmed cell death protein 1; tumor metastasis; tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response.Front Immunol. 2019 Aug 14;10:1939. doi: 10.3389/fimmu.2019.01939. eCollection 2019. Front Immunol. 2019. PMID: 31475002 Free PMC article.
-
Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine.Immunology. 2020 Jan;159(1):75-87. doi: 10.1111/imm.13126. Epub 2019 Oct 24. Immunology. 2020. PMID: 31587253 Free PMC article.
-
Nanomicelle protects the immune activation effects of Paclitaxel and sensitizes tumors to anti-PD-1 Immunotherapy.Theranostics. 2020 Jul 9;10(18):8382-8399. doi: 10.7150/thno.45391. eCollection 2020. Theranostics. 2020. PMID: 32724476 Free PMC article.
-
Immune Checkpoint Blockade in Breast Cancer Therapy.Adv Exp Med Biol. 2017;1026:383-402. doi: 10.1007/978-981-10-6020-5_18. Adv Exp Med Biol. 2017. PMID: 29282694 Review.
-
Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy.Clin Adv Hematol Oncol. 2016 Nov;14(11):922-933. Clin Adv Hematol Oncol. 2016. PMID: 27930644 Review.
References
-
- Ye L, Zhong F, Sun S, Ou X, Yuan J, Zhu J. et al. Tamoxifen induces ferroptosis in MCF-7 organoid. J Cancer Res Ther. 2023;19:1627–35. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

