Inorganic and hybrid nanomaterials for NIR-II fluorescence imaging-guided therapy of Glioblastoma and perspectives
- PMID: 40365286
- PMCID: PMC12068291
- DOI: 10.7150/thno.112204
Inorganic and hybrid nanomaterials for NIR-II fluorescence imaging-guided therapy of Glioblastoma and perspectives
Abstract
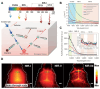
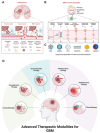
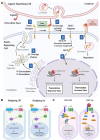
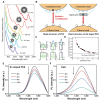
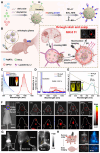
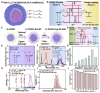
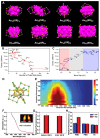
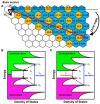
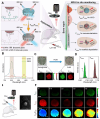
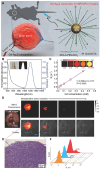
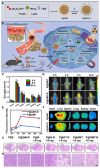
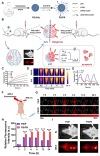
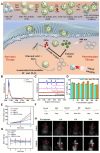
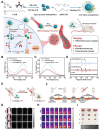
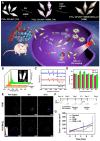
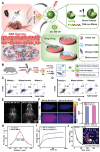
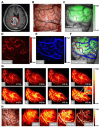
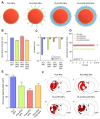
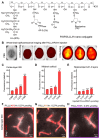
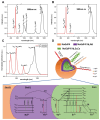
Glioblastoma (GBM) is the most invasive and lethal brain tumor, with limited therapeutic options due to its highly infiltrative nature, resistance to conventional therapies, and blood-brain barriers. Recent advancements in near-infrared II (NIR-II) fluorescence imaging have facilitated greater tissue penetration, improved resolution, and real-time visualization of GBM, providing a promising approach for precise diagnosis and treatment. The inorganic and hybrid NIR-II fluorescent materials have developed rapidly for NIR-II fluorescence imaging-guided diagnosis and therapy of many diseases, including GBM. Herein, we offer a timely update to explore the contribution of inorganic/hybrid NIR-II fluorescent nanomaterials, such as quantum dots, rare-earth-doped nanoparticles, carbon-based nanomaterials, and metal nanoclusters in imaging-guided treatment for GBM. These nanomaterials provide high photostability, strong fluorescence intensity, and tunable optical properties, allowing for multimodal imaging and enhanced therapeutic efficacy. Additionally, their integration with modern therapeutic strategies, such as photothermal therapy, chemodynamic therapy, photodynamic therapy, sonodynamic therapy, and immunotherapy, has shown significant potential in overcoming the limitations of traditional treatments. Looking forward, future advancements including safe body clearance, long-term biocompatibility, efficient BBB penetration, and extended emission wavelengths beyond 1500 nm could enhance the theranostic outcomes. The integration of dual imaging with immunotherapy and AI-driven strategies will further enhance precision and accelerate the clinical translation of smart theranostic platforms for GBM treatment.
Keywords: Glioblastoma; NIR-II fluorescence imaging; imaging-guided therapy; inorganic and hybrid nanomaterials; targeting.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Neutrophil-Targeting Semiconducting Polymer Nanotheranostics for NIR-II Fluorescence Imaging-Guided Photothermal-NO-Immunotherapy of Orthotopic Glioblastoma.Adv Sci (Weinh). 2024 Oct;11(39):e2406750. doi: 10.1002/advs.202406750. Epub 2024 Aug 19. Adv Sci (Weinh). 2024. PMID: 39159216 Free PMC article.
-
Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment.Nanoscale. 2024 Dec 5;16(47):21697-21730. doi: 10.1039/d4nr03058c. Nanoscale. 2024. PMID: 39508492 Review.
-
Genetically Engineered IL12/CSF1R-Macrophage Membrane-Liposome Hybrid Nanovesicles for NIR-II Fluorescence Imaging-Guided and Membrane-Targeted Mild Photothermal-Immunotherapy of Glioblastoma.Adv Sci (Weinh). 2025 Jun;12(23):e2500131. doi: 10.1002/advs.202500131. Epub 2025 Apr 25. Adv Sci (Weinh). 2025. PMID: 40279543 Free PMC article.
-
Engineering of exosome-liposome hybrid-based theranostic nanomedicines for NIR-II fluorescence imaging-guided and targeted NIR-II photothermal therapy of subcutaneous glioblastoma.Colloids Surf B Biointerfaces. 2025 Jan;245:114258. doi: 10.1016/j.colsurfb.2024.114258. Epub 2024 Sep 19. Colloids Surf B Biointerfaces. 2025. PMID: 39303384
-
Recent progress in development and applications of second near-infrared (NIR-II) nanoprobes.Arch Pharm Res. 2021 Feb;44(2):165-181. doi: 10.1007/s12272-021-01313-x. Epub 2021 Feb 4. Arch Pharm Res. 2021. PMID: 33538959 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

