Promotion of Cx26 mutants located in TM4 region for membrane translocation successfully rescued hearing loss
- PMID: 40365300
- PMCID: PMC12068290
- DOI: 10.7150/thno.112225
Promotion of Cx26 mutants located in TM4 region for membrane translocation successfully rescued hearing loss
Abstract
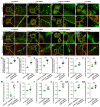
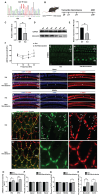
Rationale: The GJB2 gene, which encodes connexin 26 (Cx26), is recognized as the leading cause of non-syndromic hereditary hearing loss. In clinical settings, a total of 131 Cx26 mutations have been identified in association with hearing loss. Certain Cx26 mutants display normal structural and functional properties but fail to translocate to the plasma membrane. Enhancing the membrane localization of these mutants may provide a promising strategy for rescuing hearing loss and hair cell degeneration. Methods: This study investigated the membrane localization of Cx26 using in vitro cell lines, cultured cochlear explants, and in vivo murine models. Key proteins involved in the membrane localization of Cx26 were identified and validated through immunoprecipitation-mass spectrometry (IP-MS) and co-immunoprecipitation (Co-IP). Additionally, cell lines and murine models harboring Cx26 mutants were developed to evaluate the effects of Narciclasine on enhancing the membrane localization of these mutants, as well as its potential to rescue hearing loss. Results: The membrane localization of Cx26 was dependent on the integrity of the intracellular transport network consisting of microtubules, actin microfilaments, and the Golgi apparatus. Additionally, SPTBN1 played a significant role in this process. The transmembrane domain 4 (TM4) region exhibited a strong association with the membrane localization of Cx26, and Cx26 mutants located in TM4 region retained in the cytoplasm. Narciclasine promoted cytoskeletal development, thereby enhancing the membrane localization of Cx26 mutants retained in the cytoplasm. This process helped to reconstruct the inner ear gap junction network and rescue hearing loss and hair cell degeneration. Conclusion: These findings present that enhancing the membrane localization of Cx26 mutants can significantly improve auditory function. This strategy offers a potential therapeutic approach for addressing hereditary sensorineural hearing loss associated with GJB2 mutations.
Keywords: Cx26; SPTBN1; cytoskeleton; hearing loss; mutants; treatment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

