Discovery of Thieno[3,2- b]pyridine-5-carboxamide and 2,3-Difluorobenzamide Negative Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5
- PMID: 40365396
- PMCID: PMC12067350
- DOI: 10.1021/acsmedchemlett.5c00119
Discovery of Thieno[3,2- b]pyridine-5-carboxamide and 2,3-Difluorobenzamide Negative Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5
Abstract
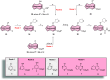
This Letter describes the discovery of novel mGlu5 NAMs VU6031545 and VU6024945. Starting from previously reported picolinamide compounds, a structure-activity relationship study of various core isosteres was conducted, leading to the identification of thieno[3,2-b]pyridine-5-carboxamide and 2,3-difluorobenzamide as competent core replacements. These compounds are highly potent as well as brain penetrant with an IVIVC agreement and improved oral bioavailability in rats.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors hold IP on mGlu5 NAMs.
Figures
References
-
- Melancon B. J.; Hopkins C. R.; Wood M. R.; Emmitte K. A.; Niswender C. M.; Christopoulos A.; Conn P. J.; Lindsley C. W. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 2012, 55, 1445–64. 10.1021/jm201139r. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

