Protecting and rejuvenating ageing skin by regulating endogenous hyaluronan metabolism using adipose-derived stem cell-secreted siRNAs
- PMID: 40365494
- PMCID: PMC12069053
- DOI: 10.3389/fmed.2025.1529936
Protecting and rejuvenating ageing skin by regulating endogenous hyaluronan metabolism using adipose-derived stem cell-secreted siRNAs
Abstract
Background: Loss of moisture is the primary cause of skin ageing and dysfunction. The skin's hydration largely depends on hyaluronan (HA) and its ability to retain water. Ultraviolet (UV) irradiation, which accounts for 80% of skin ageing (commonly referred to as photoaging), gradually disrupts the balance of HA metabolism, leading to a reduction in HA levels, dehydration, and, ultimately, the formation of wrinkles.
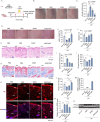
Methods: In this study, we develop an RNAi-based strategy to treat aged skin by modulating endogenous HA metabolism. Hyaluronidase 2 (HYAL2), an enzyme responsible for HA degradation, is selected as the therapeutic target, given its significant upregulation in photoaged skin. To deliver the siRNA targeting HYAL2 to the skin, human adipose-derived stem cells (ADSCs) are engineered to stably express and secrete HYAL2-targeting siRNAs (ADSC/siRH) via small extracellular vesicles (sEVs).
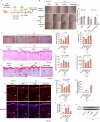
Results: In vitro experiments demonstrate that ADSC-delivered siRNAs are successfully internalised by recipient cells, where they restore UV-induced HA reduction by inhibiting HYAL2 expression. In vivo experiments revealed that subcutaneous implantation of engineered ADSCs prior to UV exposure significantly protects mouse skin from accelerated HA degradation, helping to retain water content and prevent UV-induced dryness. Furthermore, the application of engineered ADSCs to aged mouse skin can markedly restore HA and water content, effectively smoothing deep wrinkles and improving skin appearance.
Conclusion: We developed an effective biological strategy to combat skin ageing and damage by preserving endogenous HA levels, which could be applied for facial rejuvenation in the future.
Keywords: adipose-derived stem cells (ADSCs); extracellular vesicles (EVs); hyaluronan (HA); siRNA therapy; skin aging.
Copyright © 2025 Sun, He, Zhang, Liu, Chen, Pan, Fang, Wang, Jiang, Liu, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation.Stem Cell Res Ther. 2020 Jul 1;11(1):264. doi: 10.1186/s13287-020-01777-6. Stem Cell Res Ther. 2020. PMID: 32611371 Free PMC article.
-
Hyaluronan size alters chondrogenesis of adipose-derived stem cells via the CD44/ERK/SOX-9 pathway.Acta Biomater. 2018 Jan 15;66:224-237. doi: 10.1016/j.actbio.2017.11.025. Epub 2017 Nov 8. Acta Biomater. 2018. PMID: 29128538
-
Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging.Int J Mol Sci. 2022 Dec 6;23(23):15390. doi: 10.3390/ijms232315390. Int J Mol Sci. 2022. PMID: 36499715 Free PMC article.
-
Role of HYBID (Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization), Alias KIAA1199/CEMIP, in Hyaluronan Degradation in Normal and Photoaged Skin.Int J Mol Sci. 2019 Nov 19;20(22):5804. doi: 10.3390/ijms20225804. Int J Mol Sci. 2019. PMID: 31752258 Free PMC article. Review.
-
The Therapeutic Role of ADSC-EVs in Skin Regeneration.Front Med (Lausanne). 2022 Jun 9;9:858824. doi: 10.3389/fmed.2022.858824. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35755023 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

