Escherichia coli aggravates inflammatory response in mice oral mucositis through regulating Th17/Treg imbalance
- PMID: 40365536
- PMCID: PMC12069327
- DOI: 10.3389/fcimb.2025.1585020
Escherichia coli aggravates inflammatory response in mice oral mucositis through regulating Th17/Treg imbalance
Abstract
Introduction: Microbial dysbiosis links to mucosal immune dysregulation, but the specific bacterial contributions to oral mucosal inflammation remain unclear. Escherichia coli (E. coli), a pathogen well-characterized in mucosal immunity and immune regulation studies, has been observed to be enriched in chronic oral inflammatory lesions and was reported to modulate T helper 17 cells (Th17)/T regulatory cells (Treg) homeostasis. Here, we developed an oral mucositis mouse model via tongue scratch and E. coli topical application to investigate its role in Th17/Treg imbalance.
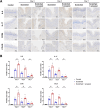
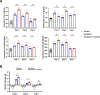
Methods: The inflammatory infiltration was evaluated by macroscopic photography and HE staining. The expression of inflammatory factors in tongue tissue and peripheral blood of mice were detected by immunohistochemical staining and enzyme-linked immunosorbent assay. The number of Th17 and Treg in mice spleen lymphocytes were evaluated with flow cytometry. Differential gene expression analysis, functional enrichment analysis and immune infiltration analysis were performed using RNA-seq data from oral lichen planus (OLP).
Results: E. coli stimulation aggravated inflammatory responses induced by scratching in lingual mucosa of mice, including increased local and systemic expression of interleukin 6 (IL6), interleukin 17 (IL17), chemokine receptor 6 (CCR6) and chemokine C-C motif ligand 20 (CCL20), increased proportions of Th17 cells and increased Th17/Treg ratio in spleen lymphocytes. Analysis of RNA-seq data from OLP revealed alterations in antimicrobial responses and inflammatory factors associated with upregulation of Th17/Treg balance.
Conclusion: This study supports the role of E. coli in promoting oral mucosal inflammation and provides an experimental basis for in vivo study of OLP from the perspective of microorganisms.
Keywords: Escherichia coli; Th17/Treg response; mice model; mucosal immunity; oral mucosal immune inflammation.
Copyright © 2025 Liu, Xia, Cheng, Geng, Li and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
E. coli LPS/TLR4/NF-κB Signaling Pathway Regulates Th17/Treg Balance Mediating Inflammatory Responses in Oral Lichen Planus.Inflammation. 2023 Jun;46(3):1077-1090. doi: 10.1007/s10753-023-01793-7. Epub 2023 Apr 5. Inflammation. 2023. PMID: 37017858
-
Escherichia coli enhances Th17/Treg imbalance via TLR4/NF-κB signaling pathway in oral lichen planus.Int Immunopharmacol. 2023 Jun;119:110175. doi: 10.1016/j.intimp.2023.110175. Epub 2023 Apr 13. Int Immunopharmacol. 2023. PMID: 37058754
-
Induction of Oral Lichen Planus-like Histopathology in Mice.J Dent Res. 2025 Mar;104(3):320-329. doi: 10.1177/00220345241304760. Epub 2024 Dec 23. J Dent Res. 2025. PMID: 39711157
-
Role of distinct CD4(+) T helper subset in pathogenesis of oral lichen planus.J Oral Pathol Med. 2016 Jul;45(6):385-93. doi: 10.1111/jop.12405. Epub 2015 Dec 23. J Oral Pathol Med. 2016. PMID: 26693958 Review.
-
Th17/Treg Cell Imbalance May Contribute to Spontaneous Preterm Labor.J Immunol Res. 2025 May 22;2025:8405365. doi: 10.1155/jimr/8405365. eCollection 2025. J Immunol Res. 2025. PMID: 40443571 Free PMC article. Review.
References
-
- Araújo R. P. N., Da Silva Freitas F. V., Nunes D. B., Da Silva Brito A. K., Da Costa D. S., De Sousa D. P., et al. . (2024). Investigating the pharmacological potential of phytol on experimental models of gastric ulcer in rats. Naunyn Schmiedebergs Arch. Pharmacol. 397, 7757–7766. doi: 10.1007/s00210-024-03085-9 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

