Tirzepatide and health-related quality of life in adults with obesity or overweight: Results from the SURMOUNT-3 phase 3 randomized trial
- PMID: 40365662
- PMCID: PMC12232368
- DOI: 10.1111/dom.16463
Tirzepatide and health-related quality of life in adults with obesity or overweight: Results from the SURMOUNT-3 phase 3 randomized trial
Abstract
Aims: Tirzepatide reduced weight significantly more than placebo in adults with obesity/overweight who had already achieved ≥5% weight reduction with a 12-week intensive lifestyle intervention (randomized population) in SURMOUNT-3, a phase 3, 72-week, randomized, double-blind clinical trial. This analysis evaluated health-related quality of life (HRQoL) with tirzepatide versus placebo treatment in the SURMOUNT-3 randomized population and selected subgroups.
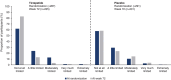
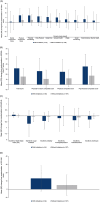
Materials and methods: The randomized population received placebo (N = 292) or tirzepatide maximum tolerated dose (N = 287) for 72 weeks. HRQoL was assessed from randomization to week 72 using the Short Form-36 Version 2 Health Survey acute form, Impact of Weight on Quality of Life-Lite Clinical Trials Version, 5-level EQ-5D version Health State Index, EQ visual analogue scale and the Patient Global Impression of Status (PGIS) for Physical Activity. In tirzepatide recipients, changes in HRQoL scores from randomization to week 72 were descriptively summarized by achievement of weight reduction thresholds, and for those with versus without physical function limitations at randomization (identified with PGIS for Physical Activity).
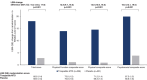
Results: Tirzepatide was associated with significantly larger improvements than placebo in most HRQoL measures from randomization to 72 weeks. Improvements in tirzepatide recipients were generally numerically larger in those who met greater weight reduction thresholds. HRQoL score changes showed greater improvements for adults with versus without physical function limitations for all measures.
Conclusions: Tirzepatide improved HRQoL in adults with obesity/overweight and was generally associated with larger improvements in adults meeting greater weight reduction thresholds and in adults with versus without reported physical function limitations at randomization.
Plain language summary: What is the context and purpose of this research study? In a clinical trial called SURMOUNT-3, tirzepatide was significantly better than placebo for reducing weight in adults with obesity/overweight who had already lost ≥5% weight following a 12-week intensive lifestyle programme. This analysis looked at the effects of tirzepatide compared with placebo for 72 weeks on quality of life (QoL) in SURMOUNT-3. In addition, among tirzepatide recipients, the relationship between meeting different weight reduction thresholds and changes in QoL was described as was the association of tirzepatide with QoL in study participants who had physical limitations at randomization. What was done? Eligible adults with obesity/overweight were enrolled in a 12-week intensive lifestyle programme. Those who lost ≥5% of initial weight at the end of the 12 weeks were invited to continue participating in the study and were randomly assigned to receive injections of either their maximum tolerated dose of tirzepatide (10 or 15 mg) or placebo for 72 weeks via single-dose pens. QoL was measured using a number of well-established surveys that assessed general health, the impact of weight on QoL and the impact of health on the level of physical ability in day-to-day life. These surveys were completed by the study participants when they were first assigned treatment with tirzepatide or placebo (randomization) and again after 72 weeks of treatment. The difference in scores from randomization to week 72 was then calculated to determine whether or not the QoL of participants had improved with treatment. In addition, in participants who took tirzepatide, changes in QoL scores from randomization to week 72 were summarized by weight reduction thresholds (≥5%, ≥10%, ≥15%, ≥20%, ≥25%), and for those with versus without physical limitations at randomization. What were the main results? Adults with obesity/overweight who had already lost ≥5% weight with a 12-week intensive lifestyle programme who then took tirzepatide for 72 weeks not only had significantly more weight loss compared with those taking placebo, but they also had significantly improved QoL. These improvements in QoL were generally larger with greater weight loss and in adults who reported physical limitations at randomization compared to those who reported no such limitations. The improvements in QoL were observed in physical function as well as in general mental health and weight-related psychological and social functions. What is the originality and relevance of this study? Findings of this study show that in addition to reducing weight in people who have already lost ≥5% weight after lifestyle interventions, tirzepatide improved QoL. This is important because other studies have shown that people with obesity have reduced QoL. Tirzepatide was generally associated with improved QoL the most in adults who had greater weight loss and in adults who reported physical limitations at randomization.
Keywords: obesity; overweight; phase 3; quality of life; tirzepatide; weight reduction.
© 2025 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Theresa Hunter Gibble: Employee and stockholder, Eli Lilly and Company. Dachuang Cao: Employee and stockholder, Eli Lilly and Company. Tammy D. Forrester: Employee and stockholder, Eli Lilly and Company. Julia Fraseur Brumm: Employee and stockholder, Eli Lilly and Company. Ariana Chao: Support for the current manuscript, Eli Lilly and Company; consulting fees, Eli Lilly and Company, Boehringer Ingelheim; grant support, on behalf of the University of Pennsylvania WW (Weight Watchers); support for attending meetings and/or travel, Eli Lilly and Company.
Figures
Similar articles
-
Indirect comparative efficacy and safety of tirzepatide 10 and 15 mg versus semaglutide 2.4 mg for the management of obesity and overweight in patients with type 2 diabetes.Diabetes Obes Metab. 2025 Sep;27(9):4709-4719. doi: 10.1111/dom.16508. Epub 2025 Jun 19. Diabetes Obes Metab. 2025. PMID: 40537987 Free PMC article. Clinical Trial.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Association between weight reduction achieved with tirzepatide and quality of life in adults with obesity: Results from the SURMOUNT-1 study.Diabetes Obes Metab. 2025 Feb;27(2):539-550. doi: 10.1111/dom.16046. Epub 2024 Nov 4. Diabetes Obes Metab. 2025. PMID: 39497468 Free PMC article. Clinical Trial.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Long-term (68 weeks) administration of nemolizumab and topical corticosteroids for prurigo nodularis in patients aged ≥ 13 years: efficacy and safety data from a phase II/III study.Br J Dermatol. 2025 Jun 20;193(1):56-65. doi: 10.1093/bjd/ljaf045. Br J Dermatol. 2025. PMID: 40112876 Clinical Trial.
References
-
- World Health Organization . Obesity and overweight . Accessed August 8, 2023. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

