Novel Aortic Dissection Model Links Endothelial Dysfunction and Immune Infiltration
- PMID: 40365676
- PMCID: PMC12175827
- DOI: 10.1161/CIRCRESAHA.125.326230
Novel Aortic Dissection Model Links Endothelial Dysfunction and Immune Infiltration
Abstract
Background: Aortic dissection (AD) is the separation of medial layers of the aorta and is a major cause of death in patients with connective tissue disorders such as Marfan syndrome. However, molecular triggers instigating AD, its temporospatial progression, and how vascular cells in each vessel layer interact and participate in the pathological process remain incompletely understood. To unravel the underlying molecular mechanisms of AD, we generated a spontaneous AD mouse model.
Methods: We incorporated a novel missense variant (p.G234D) in FBN1, the gene for fibrillin-1, identified in a patient with nonsyndromic familial AD into mice using the CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) system. We performed molecular pathological analyses of the aortic lesions by histology, immunofluorescence staining, electron microscopy, synchrotron-based imaging, and single-cell RNA sequencing. Biochemical analysis was performed to examine the binding capacity of mutant human FBN1G234D (fibrillin 1 Gly234Asp) protein to LTBPs (latent TGFβ [transforming growth factor-beta] binding proteins), and signaling pathways in the mutant aortic wall were examined by the Western blot analysis.
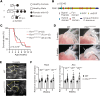
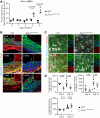
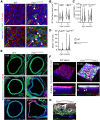
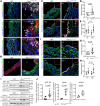
Results: Fifty percent of the Fbn1G234D/G234D mutant mice died within 5 weeks of age from multiple intimomedial tears that expanded longitudinally and progressed to aortic rupture accompanied by massive immune cell infiltration. Fbn1G234D/G234D endothelial cells exhibited altered mechanosensing with loss of parallel alignment to blood flow and upregulation of VCAM-1 (vascular cell adhesion molecule-1) and ICAM-1 (intercellular adhesion molecule-1) as early as 1 week of age. Single-cell RNA sequencing, validated by immunostaining, revealed a cluster of monocyte/macrophage predominantly in the intima at 3 weeks of age before the dissection, and the second cluster of macrophages increased during the progression of intimomedial tears, exhibiting strong CCR2+ (C-C motif chemokine receptor 2 positive) and both M1- and M2-like features. Consistently, upregulation of MMP2/9 (matrix metalloproteinase 2 and 9) was observed. Biochemically, FBN1G234D lost the ability to bind to LTBP-1, -2, and -4, resulting in the downregulation of TGFβ signaling in the aortic wall.
Conclusions: We show that interactions involving endothelial cells and macrophages/monocytes in the intima, where the extracellular matrix (ECM) microenvironment contains reduced TGFβ signaling, contribute to the initiation of AD. Our novel AD mouse model provides a unique opportunity to identify target molecules involved in the intimomedial tears that can be utilized for the development of therapeutic strategies.
Keywords: Marfan syndrome; aortic dissection; endothelial cells; fibrillin-1; macrophages.
Conflict of interest statement
None.
Figures



Comment in
-
Endothelium: A Barrier Against Aortic Dissection.Circ Res. 2025 Jun 20;137(1):43-45. doi: 10.1161/CIRCRESAHA.125.326722. Epub 2025 Jun 19. Circ Res. 2025. PMID: 40536938 No abstract available.
References
-
- Nienaber CA, Clough RE, Sakalihasan N, Suzuki T, Gibbs R, Mussa F, Jenkins MP, Thompson MM, Evangelista A, Yeh JS, et al. Aortic dissection. Nat Rev Dis Primers. 2016;2:16071. doi: 10.1038/nrdp.2016.71 - PubMed
-
- Tamori Y, Akutsu K, Kasai S, Sakamoto S, Okajima T, Yoshimuta T, Yokoyama N, Ogino H, Higashi M, Nonogi H, et al. Coexistent true aortic aneurysm as a cause of acute aortic dissection. Circ J. 2009;73:822–825. doi: 10.1253/circj.cj-08-0427 - PubMed
-
- Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331–348. doi: 10.1038/s41569-020-00472-6 - PubMed
-
- Murray CA, Edwards JE. Spontaneous laceration of ascending aorta. Circulation. 1973;47:848–858. doi: 10.1161/01.cir.47.4.848 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

