Establishment of an in vitro implantation model using a newly developed mouse endometrial organoid
- PMID: 40365775
- PMCID: PMC12091871
- DOI: 10.1242/dev.204461
Establishment of an in vitro implantation model using a newly developed mouse endometrial organoid
Abstract
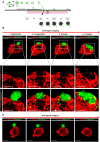
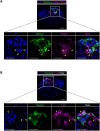
Implantation failure is a major cause of infertility, but its mechanisms remain unclear due to the lack of techniques for constructing organized endometrial structures and recapitulating the implantation process in vitro. Endometrial organoids have recently been developed, but they consist of only epithelial cells, and their apical surface faces inward, preventing blastocyst attachment. We developed an apical-out mouse endometrial organoid incorporating epithelial and stromal cells, and examined its ability to recapitulate implantation with mouse blastocysts. Mouse uteri were digested with collagenase and cultured in monolayers. The resulting aggregates were then transferred to low-attachment plates for 3D culture. After 7 days, self-organized aggregates contained E-cadherin-positive epithelial cells outside and vimentin-positive stromal cells inside. Mucin 1 signals were observed on the apical side of epithelial cells, confirming the apical-out orientation. Organoids were stimulated with sex steroid hormones and co-cultured with blastocysts. Time-lapse imaging revealed the four implantation steps: blastocyst attachment, epithelial invagination, entosis and invasion. Invaded cells expressed proliferin while surrounding stromal cells expressed cyclooxygenase 2, indicating trophoblast differentiation and decidualization. This novel organoid closely recapitulates the mouse endometrium and implantation process in vitro.
Keywords: Apical-out; Decidualization; Endometrial organoid; Implantation.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Boretto, M., Cox, B., Noben, M., Hendriks, N., Fassbender, A., Roose, H., Amant, F., Timmerman, D., Tomassetti, C., Vanhie, A.et al. (2017). Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development (Camb.) 144, 1775-1786. 10.1242/dev.148478 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

