MTFR2-Mediated Fission Drives Fatty Acid and Mitochondrial Co-Transfer from Hepatic Stellate Cells to Tumor Cells Fueling Oncogenesis
- PMID: 40365837
- PMCID: PMC12199435
- DOI: 10.1002/advs.202416419
MTFR2-Mediated Fission Drives Fatty Acid and Mitochondrial Co-Transfer from Hepatic Stellate Cells to Tumor Cells Fueling Oncogenesis
Abstract
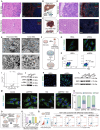
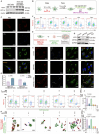
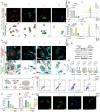
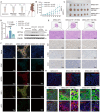
The tumor margin of hepatocellular carcinoma (HCC) is a critical zone where cancer cells invade the surrounding stroma, exhibiting unique and more invasive metabolic and migratory features compared to the tumor center, driving tumor expansion beyond the primary lesion. Studies have shown that at this critical interface, HCC cells primarily rely on fatty acid oxidation to meet their energy demands, although the underlying mechanisms remain unclear. This study demonstrates that activated hepatic stellate cells (HSCs) at the tumor margin play a pivotal role in sustaining the metabolic needs of HCC cells. Specifically, it is discovered that mitochondrial fission regulator 2 (MTFR2) in HSCs interacts with dynamin-related protein 1 (DRP1, a known mitochondrial fission machinery), preventing its lysosomal degradation, which in turn promotes mitochondrial fission. This MTFR2-driven mitochondrial fission enhances the transfer of both fatty acids and mitochondria to HCC cells, supplying essential metabolic substrates and reinforcing the mitochondrial machinery critical for tumor growth. The findings suggest that targeting MTFR2-driven mitochondrial fission may offer a novel therapeutic avenue for interfering with the metabolic crosstalk between tumor cells and the stromal niche.
Keywords: fatty acid transfer; hepatic stellate cell; hepatocellular carcinoma; mitochondrial dynamics; mitochondrial transfer.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Yang X., Yang C., Zhang S., Geng H., Zhu A. X., Bernards R., Qin W., Fan J., Wang C., Gao Q., Cancer Cell 2024, 42, 180; - PubMed
- b) Faubert B., Solmonson A., DeBerardinis R. J., Science 2020, 368, 6487; - PMC - PubMed
- c) Ji A. L., Rubin A. J., Thrane K., Jiang S., Reynolds D. L., Meyers R. M., Guo M. G., George B. M., Mollbrink A., Bergenstråhle J., Larsson L., Bai Y., Zhu B., Bhaduri A., Meyers J. M., Rovira‐Clavé X., Hollmig S. T., Aasi S. Z., Nolan G. P., Lundeberg J., Khavari P. A., Cell 2020, 182, 497; - PMC - PubMed
- d) Yang M., Song X., Zhang F., Li M., Chang W., Wang Z., Li M., Shan H., Li D., Hepatology 2024, 81, 1452. - PubMed
-
- Sun Y., Wu P., Zhang Z., Wang Z., Zhou K., Song M., Ji Y., Zang F., Lou L., Rao K., Wang P., Gu Y., Gu J., Lu B., Chen L., Pan X., Zhao X., Peng L., Liu D., Chen X., Wu K., Lin P., Wu L., Su Y., Du M., Hou Y., Yang X., Qiu S., Shi Y., Sun H., et al., Cancer Cell 2024, 42, 135. - PubMed
-
- Sung J. Y., Cheong J. H., Cells 2022, 11, 2373.
-
- a) Saha T., Dash C., Jayabalan R., Khiste S., Kulkarni A., Kurmi K., Mondal J., Majumder P. K., Bardia A., Jang H. L., Sengupta S., Nat. Nanotechnol. 2022, 17, 98; - PMC - PubMed
- b) Borcherding N., Brestoff J. R., Nature 2023, 623, 283; - PMC - PubMed
- c) Melwani P. K., Pandey B. N., Biochim. Biophys, Acta, Rev. Cancer 2023, 1878, 189028. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
