GmFER1, a soybean ferritin, enhances tolerance to salt stress and root rot disease and improves soybean yield
- PMID: 40365869
- PMCID: PMC12310874
- DOI: 10.1111/pbi.70102
GmFER1, a soybean ferritin, enhances tolerance to salt stress and root rot disease and improves soybean yield
Abstract
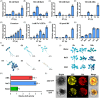
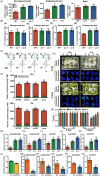
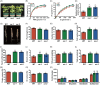
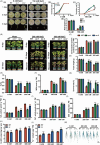
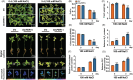
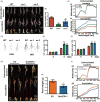
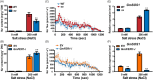
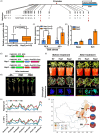
The plant stress response mechanism is activated by biotic and abiotic stresses, but its continuous activation typically affects growth. The role of ferritin in regulating biomass accumulation has been extensively characterized in diverse plant species; however, the underlying mechanisms through which it contributes to salt stress tolerance and Fusarium resistance remain poorly understood. Here, we confirm that overexpression of ferritin leads to iron accumulation and Fe3+ sequestration in both aboveground and roots, activating the iron uptake and transport system. More importantly, GmFER1 enhances salt stress tolerance and Fusarium resistance. First, GmFER1 is localized in chloroplasts and significantly induced by salt stress and Fusarium infection. Overexpression of GmFER1 increases soybean yield per plant by enhancing net photosynthetic rate and Rubisco enzyme activity, without activating the reactive oxygen scavenging mechanism. Under salt stress, GmFER1 enhances resistance by improving the activities of SOD and CAT enzymes, as well as Na+ efflux capacity. Under Fusarium infection, GmFER1 enhances resistance to the pathogen by boosting antioxidant capacity. Moreover, iron-deficiency tests revealed that increased CAT and SOD activities under salt stress are linked to iron ions accumulation. Lastly, we analysed the effects of GmFER1 gene variation on salt tolerance, disease resistance and 23 agronomic traits related to yield and quality. Further analysis of GmFER1 gene variation revealed that the Hap2 haplotypes could potentially enhance salt resistance, disease resistance, pod number and oil content in soybean. Our research offers a new way to reduce growth penalties while boosting plant resistance to salt stress and Fusarium infection.
Keywords: Fe; NMT (non‐invasive micro‐test technology); ROS (reactive oxygen species); salt stress; soybean ferritin.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Aoki, T. , O'Donnell, K. and Scandiani, M.M. (2005) Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp. nov., F. cuneirostrum sp. nov., F. tucumaniae, and F. virguliforme . Mycoscience 46, 162–183.
-
- Begara‐Morales, J.C. , Sánchez‐Calvo, B. , Chaki, M. , Mata‐Pérez, C. , Valderrama, R. , Padilla, M.N. , López‐Jaramillo, J. et al. (2015) Differential molecular response of monodehy droascorbate reductase and glutathione reductase by nitration and S‐nitrosylation. J. Exp. Bot. 66, 5983–5996. - PMC - PubMed
-
- Bradbury, P.J. , Zhang, Z. , Kroon, D.E. , Casstevens, T.M. , Ramdoss, Y. and Buckler, E.S. (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. - PubMed
-
- Briat, J.‐F. and Lobréaux, S. (1997) Iron transport and storage in plants. Trends Plant Sci. 2, 187–193.
-
- Briat, J.‐F. , Dubos, C. and Gaymard, F. (2015a) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 20, 33–40. - PubMed
MeSH terms
Substances
Grants and funding
- U22A20473/National Natural Science Foundation of China
- LH2023C004/Heilongjiang Provincial Project
- 2024ZXDXB54/Heilongjiang Provincial Project
- TD2022C003/Heilongjiang Provincial Project
- ZD2022C002/Heilongjiang Provincial Project
- 2021YFF1001204/National Key Research and Development Program of China
- 2021YFD1201604/National Key Research and Development Program of China
- 2021YFD1201103/National Key Research and Development Program of China
- 2023ZD04036/National project of soybean biological breeding
- CARS-04-PS07/National Ten-thousand Talents Program, The national project
- NEAU2023QNLJ-003/Young leading talents of Northeast Agricultural University
LinkOut - more resources
Full Text Sources
Miscellaneous

