Effect of creatine monohydrate on motor function in children with facioscapulohumeral muscular dystrophy: A multicenter, randomized, double-blind placebo-controlled crossover trial
- PMID: 40366059
- PMCID: PMC12149790
- DOI: 10.1002/phar.70025
Effect of creatine monohydrate on motor function in children with facioscapulohumeral muscular dystrophy: A multicenter, randomized, double-blind placebo-controlled crossover trial
Abstract
Background: Facioscapulohumeral muscular dystrophy (FSHD) is a rare, progressive muscle disease with no available disease-modifying therapy. Creatine monohydrate (CrM) has been shown to improve muscle strength in individuals with muscular dystrophies but has not been tested in young people with FSHD. This study aimed to explore the efficacy of CrM on motor function in children with FSHD.
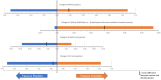
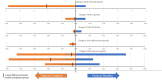
Methods: In a randomized placebo-controlled double-blind crossover trial, powdered CrM at a dose of 100 mg/kg/day (maximum 10 g daily) was compared with placebo in two 12-week treatment periods with a 6-week washout between crossover arms. The primary outcome measure was the Motor Function Measure for Neuromuscular Disease (MFM-32) with secondary outcomes assessing safety, endurance, strength, patient-reported outcome measures, and muscle morphology measurements as assessed by whole-body magnetic resonance imaging (MRI).
Results: Thirteen children were enrolled (mean (standard deviation, SD) 12.2 (2.67) years of age) and 11 patients completed both trial treatment periods. In an intention-to-treat analysis, no clinically meaningful difference was seen between treatment groups as measured by the mean difference in MFM-32 (0.19, 95% confidence interval (CI) -0.71 to 1.08). However, there was an improvement in 6-minute walk distance of 27.74 m (95% CI -1.41 to 56.88) and trends to improvement in the FSHD-Composite Outcome Measure for Pediatrics (FSHD-COM Peds), 10 meter walk/run, and in MRI measures. There were no serious adverse events. Serum creatinine increased by a mean 12.63 μmol/L (95% CI 1.14 to 24.12) post-CrM treatment, though this was presumed to reflect increased creatinine production. No participants discontinued CrM due to adverse events.
Conclusion: CrM is safe and well tolerated in children with FSHD. Although CrM had no effect on motor function as measured by the MFM-32 compared with placebo, there were trends toward improvement in the 6-minute walk distance and other secondary outcome measures. This study confirms the feasibility of conducting clinical trials in children with FSHD. Further assessment of the efficacy of CrM in pediatric FSHD is warranted in a larger randomized controlled clinical trial. Future studies may benefit from stratifying population cohorts according to functional ability or by MRI fat infiltration measurements.
Keywords: FSHD; creatine monohydrate; neuromuscular; pediatrics.
© 2025 The Author(s). Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy published by Wiley Periodicals LLC on behalf of ACCP Foundation, Ltd.
Conflict of interest statement
Dr. Woodcock has received honoraria for work performed, including educational activities and attendance at advisory board meetings from pharmaceutical companies Avidity, Biogen, Novartis, Roche, and Solid Biosciences. Dr. Woodcock has received grants for research work from FSHD Global Research Foundation, FSHD Society, Fulcrum Therapeutics, and the NIH. Dr. Woodcock has been the principal investigator on a number of industry‐sponsored clinical trials, including Biogen, Catabasis, Dyne Therapeutics, EryDel, Novartis, Percheron Therapeutics, Pfizer, PTC, Roche, and Sarepta. None of these disclosures affected the work Dr. Woodcock performed on this clinical trial. Dr. Ryan has since left the above affiliations to take up public office. Dr. Davidson has received research grant funding from Pfizer. Dr. Yiu has received advisory board honoraria from Biogen and Roche, and has received research support from Biogen, Roche, Pfizer, and PTC therapeutics unrelated to the content of this manuscript. Dr. Yiu has been the principal investigator on a number of industry‐sponsored clinical trials. None of these disclosures affected the work Dr. Yiu performed on this clinical trial. Prof. Delatycki has received grant awards from NHMRC and is the principal investigator in industry‐sponsored clinical trials including trials sponsored by Rearta and PTC. All other authors report no conflicts of interest.
Figures

References
-
- Woodcock IR, Fraser L, Norman P, Pysden K, Manning S, Childs AM. The prevalence of neuromuscular disease in the paediatric population in Yorkshire, UK: variation by ethnicity and deprivation status. Dev Med Child Neurol. 2016;58(8):877‐883. - PubMed
-
- Goselink RJM, van Kernebeek CR, Mul K, et al. A 22‐year follow‐up reveals a variable disease severity in early‐onset facioscapulohumeral dystrophy. Eur J Paediatr Neurol. 2018;22(5):782‐785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

