Loss of LasR function leads to decreased repression of Pseudomonas aeruginosa PhoB activity at physiological phosphate concentrations
- PMID: 40366151
- PMCID: PMC12186492
- DOI: 10.1128/jb.00189-24
Loss of LasR function leads to decreased repression of Pseudomonas aeruginosa PhoB activity at physiological phosphate concentrations
Abstract
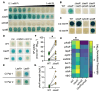
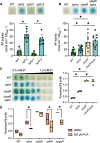
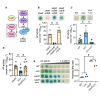
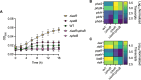
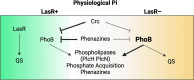
The Pseudomonas aeruginosa LasR transcription factor plays a role in quorum sensing (QS) across phylogenetically distinct lineages. However, isolates with loss-of-function mutations in lasR (LasR- strains) are commonly found in diverse settings, including infections where they are associated with worse clinical outcomes. In LasR- strains, the LasR-regulated transcription factor RhlR can also be stimulated by the activity of the two-component system PhoR-PhoB in low-inorganic phosphate (Pi) conditions. Here, we demonstrate a novel link between LasR and PhoB in which the absence of LasR increases PhoB activity at physiological Pi concentrations and increases the Pi concentration necessary for PhoB inhibition. PhoB activity was also less sensitive to repression by Pi in mutants lacking different QS regulators (RhlR and PqsR) and in mutants lacking genes required for QS-induced phenazine production, suggesting that decreased phenazine production is one reason for increased PhoB activity in LasR- strains. In addition, the CbrA-CbrB two-component system, which can be more active in LasR- strains, was necessary for increased PhoB activity in LasR- strains, and loss of the CbrA-CbrB-controlled translational repressor Crc was sufficient to activate PhoB in LasR+ P. aeruginosa. Phenazines and CbrA-CbrB affected PhoB activity independently. The ∆lasR mutant also had PhoB-dependent growth advantages in the Pi-deplete medium and increased virulence-associated gene expression at physiological Pi, in part through reactivation of QS. This work suggests PhoR-PhoB activity may contribute to the fitness and virulence of LasR- P. aeruginosa and subsequent clinical outcomes.IMPORTANCELoss-of-function mutations in the gene encoding the Pseudomonas aeruginosa quorum sensing (QS) regulator LasR occur frequently and are associated with worse clinical outcomes. We have found that LasR- P. aeruginosa have elevated PhoB activity at physiological concentrations of inorganic phosphate (Pi). PhoB activity promotes Pi acquisition as well as the expression of QS and virulence-associated genes. Previous work has shown that PhoB induces RhlR, another QS regulator, in a LasR- mutant in low-Pi conditions. Here, we demonstrate a novel relationship wherein LasR represses PhoB activity through the production of phenazines and Crc-mediated translational repression. This work suggests PhoB activity may contribute to the increased virulence of LasR- P. aeruginosa.
Keywords: CbrB; Crc; LasR; PhoB; PhoR; Pseudomonas aeruginosa; RhlR; phenazines; phosphate scavenging; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
Loss of LasR function leads to decreased repression of Pseudomonas aeruginosa PhoB activity at physiological phosphate concentrations.bioRxiv [Preprint]. 2024 May 1:2024.03.27.586856. doi: 10.1101/2024.03.27.586856. bioRxiv. 2024. Update in: J Bacteriol. 2025 Jun 24;207(6):e0018924. doi: 10.1128/jb.00189-24. PMID: 38585852 Free PMC article. Updated. Preprint.
References
-
- Bansal VK. 1990. Serum inorganic phosphorous. In HK W, HD Hall, JW Hurst (ed), Clinical methods: the history, physical, and laboratory examinations, 3rd ed. Butterworths, Boston. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

