Influenza A virus interferes with respiratory syncytial virus in mice and reconstituted human airway epithelium
- PMID: 40366152
- PMCID: PMC12131752
- DOI: 10.1128/spectrum.03187-24
Influenza A virus interferes with respiratory syncytial virus in mice and reconstituted human airway epithelium
Abstract
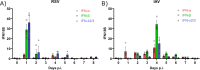
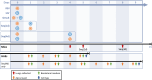
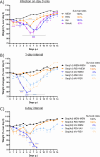
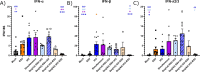
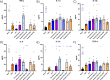
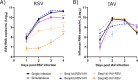
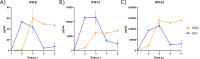
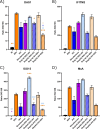
Epidemiological studies suggest that respiratory syncytial virus (RSV) and influenza A virus (IAV) might interfere with each other. Viral interference mainly relies on interferon production elicited by a first virus that reduces the replication of a second virus. In this paper, we first investigated the interactions between RSV-A2 and influenza A(H1N1)pdm09 in BALB/c mice infected with each single virus or both viruses simultaneously or sequentially before, at the peak of interferon elicited by each virus, or after that peak. IAV reduced by almost 3.0 logs the replication of RSV administered at the peak of interferon induced by influenza, but the opposite was not true. However, IAV-infected mice challenged with RSV or the vehicle lost more weight and had a lower survival rate compared to single infections. Interferon expression, cytokine levels, and pulmonary inflammation were almost similar between groups. Disease worsening was attributed to an aggravation of IAV-induced pulmonary congestion following intranasal instillation of fluid (with or without RSV). In human airway epithelia, IAV also interfered with RSV replication. Viral interference was dependent on the timing and sequence of infections but not on differential interferon susceptibilities. Overall, our results help to understand the mechanisms of the interaction between two major respiratory viruses.IMPORTANCERespiratory syncytial and influenza viruses may interfere with each other based on epidemiological studies. It is suggested that a first virus may induce the production of interferon and interfere with the replication of a second unrelated virus. Our data showed that the influenza A virus interferes with respiratory syncytial virus replication in mouse lungs, but the opposite was not observed. In reconstituted human airway epithelia, viral interference was dependent on the timing and sequence of infections but not on differential interferon susceptibilities. Understanding the mechanisms of interaction between respiratory viruses may help the development of prophylactic or therapeutic modalities.
Keywords: human airway epithelium; influenza virus; interferon; mice; respiratory syncytial virus; viral interference.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Exacerbation of Influenza A Virus Disease Severity by Respiratory Syncytial Virus Co-Infection in a Mouse Model.Viruses. 2021 Aug 18;13(8):1630. doi: 10.3390/v13081630. Viruses. 2021. PMID: 34452495 Free PMC article.
-
Influenza A(H1N1)pdm09 Virus but Not Respiratory Syncytial Virus Interferes with SARS-CoV-2 Replication during Sequential Infections in Human Nasal Epithelial Cells.Viruses. 2022 Feb 15;14(2):395. doi: 10.3390/v14020395. Viruses. 2022. PMID: 35215988 Free PMC article.
-
Inflammation and emphysema in cigarette smoke-exposed mice when instilled with poly (I:C) or infected with influenza A or respiratory syncytial viruses.Respir Res. 2016 Jul 1;17(1):75. doi: 10.1186/s12931-016-0392-x. Respir Res. 2016. PMID: 27363862 Free PMC article.
-
The Development of Animal Models for Respiratory Syncytial Virus (RSV) Infection and Enhanced RSV Disease.Viruses. 2024 Oct 30;16(11):1701. doi: 10.3390/v16111701. Viruses. 2024. PMID: 39599816 Free PMC article. Review.
-
The Story behind the Science: On the discovery of respiratory syncytial virus.mBio. 2025 Mar 12;16(3):e0307424. doi: 10.1128/mbio.03074-24. Epub 2025 Jan 21. mBio. 2025. PMID: 39835804 Free PMC article. Review.
References
-
- Pott H, J LeBlanc J, S ElSherif M, Hatchette TF, McNeil SA, Andrew MK, Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN) . 2024. Clinical features and outcomes of influenza and RSV coinfections: a report from Canadian immunization research network serious outcomes surveillance network. BMC Infect Dis 24:147. doi:10.1186/s12879-024-09033-5 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

