Drosophila melanogaster Toll-9 elicits antiviral immunity against Drosophila C virus
- PMID: 40366172
- PMCID: PMC12172494
- DOI: 10.1128/jvi.02214-24
Drosophila melanogaster Toll-9 elicits antiviral immunity against Drosophila C virus
Abstract
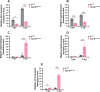
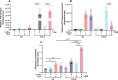
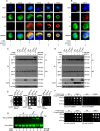
The Toll pathway plays a pivotal role in innate immune responses against pathogens. The evolutionarily conserved pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), play a crucial role in recognition of pathogen-associated molecular patterns (PAMPs). The Drosophila genome encodes nine Toll receptors that are orthologous to mammalian TLRs. While mammalian TLRs directly recognize PAMPs, most Drosophila Tolls recognize the proteolytically cleaved ligand Spätzle to activate downstream signaling cascades. In this study, we demonstrated that Toll-9 is crucial for antiviral immunity against Drosophila C virus (DCV), a natural pathogen of Drosophila. A transposable element insertion in the Toll-9 gene renders the flies more susceptible to DCV. The stable expression of Toll-9 in Drosophila S2 cells results in increased Dicer2 induction and reduced AKT phosphorylation, collectively establishing an antiviral state that inhibits DCV replication. Toll-9 localizes to endosomes, where it binds viral double-stranded RNA (dsRNA), highlighting its role in detecting viral replication intermediates. Together, these findings identify Toll-9 as a key player in antiviral immunity against DCV infection, acting through its ability to recognize dsRNA and drive Dicer2 expression, along with other AKT-mediated antiviral responses.
Importance: Insects rely on innate immunity and RNA interference (RNAi) to combat viral infections. Our study underscores the pivotal role of Drosophila Toll-9 in antiviral immunity, aligning with findings in Bombyx mori, where Toll-9 activation upregulates the RNAi component Dicer2. We demonstrate that Drosophila Toll-9 functions as a pattern recognition receptor (PRR) for double-stranded RNA (dsRNA) during Drosophila C virus (DCV) infection, akin to mammalian Toll-like receptors (TLRs). Toll-9 activation during DCV infection leads to the upregulation of Dicer2 and Argonaute2 and dephosphorylation of AKT. This study also reveals that Toll-9 localizes in endosomal compartments where it interacts with dsRNA. These insights enhance our understanding of Drosophila innate immune mechanisms, reflecting the evolutionary conservation of immune responses across diverse species and providing impetus for further research into the conserved roles of TLRs across the animal kingdom.
Keywords: AKT; DCV; Dicer2; JAK/STAT; dsRNA; endosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
Drosophila melanogaster Toll-9 elicits antiviral immunity against Drosophila C virus.bioRxiv [Preprint]. 2024 Jun 22:2024.06.19.599730. doi: 10.1101/2024.06.19.599730. bioRxiv. 2024. Update in: J Virol. 2025 Jun 17;99(6):e0221424. doi: 10.1128/jvi.02214-24. PMID: 38948804 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

