Dissection of the global responses of mandarin fish pyloric cecum to an acute ranavirus (MRV) infection reveals the formation of serositis and then ascites
- PMID: 40366173
- PMCID: PMC12172472
- DOI: 10.1128/jvi.02308-24
Dissection of the global responses of mandarin fish pyloric cecum to an acute ranavirus (MRV) infection reveals the formation of serositis and then ascites
Abstract
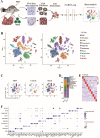
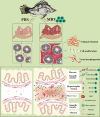
Mandarin fish ranavirus (MRV), a new member of the species Ranavirus micropterus1, sharing over 98% whole-genome nucleotide identity with the well-known largemouth bass virus (LMBV), is a distinct member of the genus Ranavirus within the family Iridoviridae. Our recent work showed that acute MRV infection predominantly affects the pyloric cecum, a critical visceral organ in mandarin fish, and was hypothesized to drive the characteristic external clinical sign of severe ascites. In this study, we reveal that acute MRV infection initially targets the serosal layer of the pyloric cecum of mandarin fish, leading to rapid progression into fibrinous serositis characterized by serosal hypertrophy, fibrosis, hyperemia, edema, and tissue adhesions. Using single-cell RNA sequencing, we dissect the cellular composition of epithelial, immune, and stromal populations, identifying significant enrichment of macrophages and granulocytes, alongside T and natural killer cells, as key mediators of acute cytokine and inflammatory responses. Then, robust experimental evidence demonstrates that MRV infects specific immune cell subsets of T and B cells and stromal cells of fibroblasts, myofibroblasts, endothelial cells, and pericytes, resulting in upregulation of genes and pathways associated with extracellular matrix (ECM) formation, collagen biosynthesis, and vascular remodeling in the hyperplastic serosal zone. Additionally, both host-derived type V collagens and MRV-encoded collagens are implicated in ECM formation in the hypertrophic serosa. Collectively, this study provides a comprehensive single-cell resolution analysis of the pyloric cecum's response to acute MRV infection and highlights virus-driven serositis as the underlying cause of severe ascites in mandarin fish.IMPORTANCEThe pyloric cecum is a vital digestive and immune organ in many bony fish species, including the mandarin fish, a carnivorous species with an exceptionally developed pyloric cecum comprising 207-326 ceca per individual. While MRV/LMBV infects various fish species, severe ascites is uniquely observed in infected mandarin fish. This study demonstrates that acute MRV infection induces fibrinous serositis in the pyloric cecum, characterized by hyperemia, edema, and hyperplasia, ultimately resulting in ascites and mortality. Leveraging single-cell RNA sequencing, we provide a detailed landscape of the cell types affected or involved in the inflammatory response, revealing their roles in the pathogenesis of serositis. These findings advance our understanding of MRV-induced pathology and its species-specific manifestations.
Keywords: ScRNA transcription; ascites syndrome; mandarin fish ranavirus (MRV); pyloric cecum; serositis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Cheng K, Jones MEB, Jancovich JK, Burchell J, Schrenzel MD, Reavill DR, Imai DM, Urban A, Kirkendall M, Woods LW, Chinchar VG, Pessier AP. 2014. Isolation of a Bohle-like iridovirus from boreal toads housed within a cosmopolitan aquarium collection. Dis Aquat Organ 111:139–152. doi: 10.3354/dao02770 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

