Endothelial cell-released mitochondrial DNA promotes B cell differentiation and virus replication during severe fever with thrombocytopenia syndrome virus infection
- PMID: 40366175
- PMCID: PMC12172443
- DOI: 10.1128/jvi.01323-24
Endothelial cell-released mitochondrial DNA promotes B cell differentiation and virus replication during severe fever with thrombocytopenia syndrome virus infection
Abstract
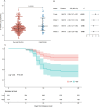
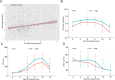
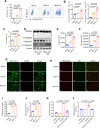
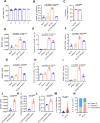
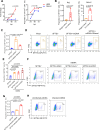
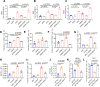
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease acquired through tick bites. We have previously demonstrated the correlation between SFTSV-induced mitochondrial dysfunction and inflammation induction, disease progression, and fatal outcome. In the current study, our clinical observation study establishes a strong correlation between elevated levels of circulating cell-free mtDNA and poor prognosis. In vivo studies further reveal endothelial cells as an important source responsible for releasing mtDNA into circulation, which promotes B cell activation, migration, and differentiation via Toll-like receptor 9 (TLR9). Notably, TLR9 activation enhances B-cell susceptibility to SFTSV infection. These findings suggest that mtDNA released by injured endothelial cells facilitates B cell differentiation and virus replication, emphasizing the significant role of mitochondrial damage within endothelial cells in contributing to the severity of SFTS outcomes.IMPORTANCESevere fever with thrombocytopenia syndrome (SFTS) is a new acute tick-borne infectious disease with a high fatality rate of 10%-50%. There is a strong correlation between SFTSV-induced mitochondrial dysfunction and inflammation induction, disease progression, and fatal outcome. Our research has revealed the crucial role of mtDNA in predicting the prognosis of SFTS and its impact on vascular endothelial injuries as well as B cell differentiation, two previously unexplored features of SFTSV infection. Moreover, mtDNA could activate the TLR9 signal to induce plasmablast differentiation in B cells and promote SFTSV infection. This study provides valuable mechanistic and clinical insights into the adverse outcomes associated with SFTSV infection.
Keywords: B cell; SFTS; TLR9; endothelial cell; mtDNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Metformin as antiviral therapy protects hyperglycemic and diabetic patients.mBio. 2025 Jun 11;16(6):e0063425. doi: 10.1128/mbio.00634-25. Epub 2025 May 20. mBio. 2025. PMID: 40391966 Free PMC article.
-
hnRNPA2B1 recognizes RNA virus SFTSV infection through mitochondrial DNA.mBio. 2025 Aug 13;16(8):e0166825. doi: 10.1128/mbio.01668-25. Epub 2025 Jul 21. mBio. 2025. PMID: 40689679 Free PMC article.
-
Age-associated immune dysregulation and B cell dysfunction drive severe outcomes in SFTSV infection.PLoS Pathog. 2025 Aug 12;21(8):e1013402. doi: 10.1371/journal.ppat.1013402. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40794649 Free PMC article.
-
Bandavirus dabieense: A review of epidemiology, clinical characteristics, pathophysiology, treatment and prevention.Virulence. 2025 Dec;16(1):2520343. doi: 10.1080/21505594.2025.2520343. Epub 2025 Jun 17. Virulence. 2025. PMID: 40519175 Free PMC article. Review.
-
Transmission of Severe Fever with Thrombocytopenia Syndrome (SFTS) to humans: A systematic review of individual participant data and meta-analysis.J Infect Public Health. 2025 Jun;18(6):102685. doi: 10.1016/j.jiph.2025.102685. Epub 2025 Jan 30. J Infect Public Health. 2025. PMID: 40073663
References
-
- Kuhn JH, Adkins S, Alioto D, Alkhovsky SV, Amarasinghe GK, Anthony SJ, Avšič-Županc T, Ayllón MA, Bahl J, Balkema-Buschmann A, et al. 2020. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch Virol 165:3023–3072. doi: 10.1007/s00705-020-04731-2 - DOI - PMC - PubMed
-
- Tran XC, Kim SH, Lee J-E, Kim S-H, Kang SY, Binh ND, Duc PV, Phuong PTK, Thao NTP, Lee W, Bae J-Y, Park M-S, Kim M, Yoo JR, Heo ST, An KH, Kim JM, Cho N-H, Kee S-H, Lee KH. 2022. Serological evidence of severe fever with thrombocytopenia syndrome virus and IgM positivity were identified in healthy residents in Vietnam. Viruses 14:2280. doi: 10.3390/v14102280 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

