Analysis of the SARS-CoV-2 inactivation mechanism using violet-blue light (405 nm)
- PMID: 40366184
- PMCID: PMC12175510
- DOI: 10.1128/aem.00403-25
Analysis of the SARS-CoV-2 inactivation mechanism using violet-blue light (405 nm)
Abstract
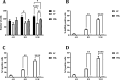
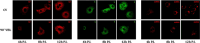
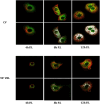
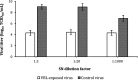
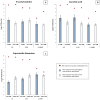
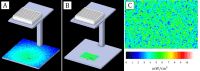
The study evaluated the effects of violet-blue light (VBL) on cell viability and replication, carbonylation of three structural proteins (S, E, and N) and one non-structural protein (NSP13), and direct damage to the RNA of SARS-CoV-2. The virus was exposed to increasing doses of VBL along with influenza A and B viruses to compare their susceptibility. At the highest dose (21.6 J/cm2), SARS-CoV-2 was significantly more susceptible to VBL than the influenza viruses, with a reduction in viral titer of 2.33 log10. Viral RNA did not show significant changes after exposure to VBL, as demonstrated by next-generation sequencing and real-time PCR quantification, suggesting that the inactivation process does not involve direct nucleic acid damage. To exclude the role of the culture suspension in the inactivation process, virus viability experiments were performed using different dilutions of Dulbecco's modified Eagle's medium (DMEM) in phosphate-buffered saline (PBS). The results indicated that the suspension medium played a secondary role in virus inactivation, as viability did not increase with increasing DMEM dilution. Subsequent tests with three different antioxidants (NAC, AsA, and SOD) at different concentrations prevented viral inactivation, from 99.99% to 85.43% (with SOD 0.003 mM). Carbonylation of S and E proteins was more pronounced when viruses were suspended in DMEM rather than PBS, although the tests demonstrated that the intrinsic properties of the viral membrane were a crucial element to consider in relation to its susceptibility to VBL.IMPORTANCELight-based disinfection methods are often used in combination with other cleaning methods due to their non-invasive nature, versatility, and environmental benefits. VBL is an effective approach as it induces the production of reactive oxygen species that reduce microbial viability. In this study, lipid peroxidation was identified as an important factor affecting the structural integrity and function of the viral envelope, reducing its ability to interact with host cells and consequently its ability to be infectious. The lipid envelope of SARS-CoV-2, composed mainly of glycerophospholipids and lacking cholesterol and sphingolipids, appears to be the critical factor in its susceptibility, distinguishing it from influenza viruses, which have a lipid profile richer in components that protect against oxidative stress.
Keywords: SARS-CoV-2 envelope; lipid peroxidation; protein carbonylation; reactive oxygen species; violet-blue light.
Conflict of interest statement
E.M. is founder and Chief Scientific Officer of VisMederi Srl and VisMederi Research Srl. The other authors declare no competing interests.
Figures

Similar articles
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
The pan-variant potential of light: 425 nm light inactivates SARS-CoV-2 variants of concern and non-cytotoxic doses reduce viral titers in human airway epithelial cells.mSphere. 2025 Jun 25;10(6):e0023025. doi: 10.1128/msphere.00230-25. Epub 2025 May 28. mSphere. 2025. PMID: 40434113 Free PMC article.
-
Comparison of the inactivation of seven foodborne pathogens and spoilage bacteria under 405 nm blue light treatment in liquid media and on solid surfaces.Microbiol Spectr. 2025 Jul;13(7):e0009325. doi: 10.1128/spectrum.00093-25. Epub 2025 May 23. Microbiol Spectr. 2025. PMID: 40407369 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
References
-
- 2024. COVID-19 deaths | WHO COVID-19 dashboard datadot. Available from: https://data.who.int/dashboards/covid19/cases
-
- Tang JW, Caniza MA, Dinn M, Dwyer DE, Heraud JM, Jennings LC, Kok J, Kwok KO, Li Y, Loh TP, Marr LC, Nara EM, Perera N, Saito R, Santillan-Salas C, Sullivan S, Warner M, Watanabe A, Zaidi SK. 2022. An exploration of the political, social, economic and cultural factors affecting how different global regions initially reacted to the COVID-19 pandemic. Interface Focus 12:20210079. doi: 10.1098/rsfs.2021.0079 - DOI - PMC - PubMed
-
- Hui DS, Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. 2020. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 91:264–266. doi: 10.1016/j.ijid.2020.01.009 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

