Fatigue Resistance and Mitochondrial Adaptations to Isometric Interval Training in Dystrophin-Deficient Muscle: Role of Contractile Load
- PMID: 40366239
- PMCID: PMC12077386
- DOI: 10.1096/fj.202500618RR
Fatigue Resistance and Mitochondrial Adaptations to Isometric Interval Training in Dystrophin-Deficient Muscle: Role of Contractile Load
Abstract
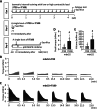
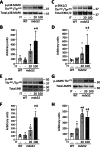
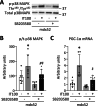
In normal mouse skeletal muscles, interval training (IT)-mimicking neuromuscular electrical stimulation enhances muscle fatigue resistance and mitochondrial content, with greater gains observed at high (100 Hz stimulation, IT100) compared to low (20 Hz stimulation, IT20) contractile load. In this study, we compared the effects of repeated IT100 and IT20 on fatigue resistance and mitochondrial adaptations in young male mdx52 mice (4- to 6-week-old), an animal model for Duchenne muscular dystrophy. Plantar flexor muscles were stimulated in vivo using supramaximal electrical stimulation to induce isometric contractions every other day for 4 weeks (a total of 15 sessions). In non-trained muscles of mdx52 mice, decreased fatigue resistance was associated with reduced citrate synthase activity, lower peroxisome proliferator-activated receptor γ coactivator 1 alpha (PGC-1α) protein expression, and diminished levels of mitochondrial respiratory chain complex II, and an increased percentage of Evans Blue dye-positive areas. IT100, but not IT20, markedly improved fatigue resistance and restored all these alterations in mdx52 mice. Furthermore, an acute session of IT100, but not IT20, led to increased phosphorylation of p38 mitogen-activated protein kinase (MAPK) and elevated mRNA levels of PGC-1α, which were blocked by the p38 MAPK inhibitor SB203580. These findings suggest that contractile load is a key determinant of isometric IT-induced improvements in fatigue resistance, even in dystrophin-deficient muscles, potentially through a p38 MAPK/PGC-1α-mediated increase in mitochondrial content.
Keywords: contractile load; fatigue resistance; isometric interval training; mitochondria; muscular dystrophy.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Frascarelli M., Rocchi L., and Feola I., “EMG Computerized Analysis of Localized Fatigue in Duchenne Muscular Dystrophy,” Muscle & Nerve 11 (1988): 757–761. - PubMed
-
- Wineinger M. A., Abresch R. T., Walsh S. A., and Carter G. T., “Effects of Aging and Voluntary Exercise on the Function of Dystrophic Muscle From mdx Mice,” American Journal of Physical Medicine & Rehabilitation 77 (1998): 20–27. - PubMed
-
- Bellissimo C. A., Delfinis L. J., Hughes M. C., et al., “Mitochondrial Creatine Sensitivity Is Lost in the D2.mdx Model of Duchenne Muscular Dystrophy and Rescued by the Mitochondrial‐Enhancing Compound Olesoxime,” American Journal of Physiology. Cell Physiology 324 (2023): C1141–C1157. - PubMed
-
- Wehling‐Henricks M., Oltmann M., Rinaldi C., Myung K. H., and Tidball J. G., “Loss of Positive Allosteric Interactions Between Neuronal Nitric Oxide Synthase and Phosphofructokinase Contributes to Defects in Glycolysis and Increased Fatigability in Muscular Dystrophy,” Human Molecular Genetics 18 (2009): 3439–3451. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

