E-Cadherin Is a Structuring Component of Invadopodia in Pancreatic Cancer
- PMID: 40366255
- PMCID: PMC12077114
- DOI: 10.1111/jcmm.70608
E-Cadherin Is a Structuring Component of Invadopodia in Pancreatic Cancer
Abstract
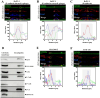
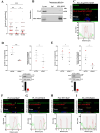
The appearance of hybrid epithelial-mesenchymal (E/M) cells expressing E-cadherin is favourable for the establishment of pro-invasive function. Although the potential role of E-cadherin in cancer invasion is now accepted, the molecular mechanisms involved in this process are not completely elucidated. To gain further insight, we focused our analysis on invadopodia formation, an early event in the invasion process. We used models of E/M hybrid cell lines, tissue sections and patient-derived xenografts from a multi-centre clinical trial. E-cadherin involvement in invadopodia formation was assessed using a gelatin-FITC degradation assay. Mechanistic studies were performed by using proteomic analysis, siRNA strategy and proximity ligation assay. We showed that E-cadherin is a critical component of invadopodia. This unexpected localization results from a synergistic trafficking of E-cadherin and MT1-MMP through a Rab vesicle-dependent pathway. Modulation of E-cadherin expression or activation impacted invadopodia formation. Moreover, colocalization of E-cadherin and Actin in "ring structures" as precursors of invadopodia reveals that E-cadherin is required for invadopodia structuration. E-cadherin, initially localised in the adherens junctions, could be recycled to nascent invadopodia where it will interact with several components enriched in invadopodia, such as Arp2/3, Cortactin or MT1-MMP. The trans-adhesive properties of E-cadherin are therefore essential for structuring invadopodia. This new localisation of E-cadherin and its unexpected role in cell invasion shine a new light on hybrid E/M transition features in tumoral invasion.
Keywords: EMT hybrid cells; adhesion molecules; cell invasion; matrix degradation.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Coronin 1C promotes triple-negative breast cancer invasiveness through regulation of MT1-MMP traffic and invadopodia function.Oncogene. 2018 Dec;37(50):6425-6441. doi: 10.1038/s41388-018-0422-x. Epub 2018 Jul 31. Oncogene. 2018. PMID: 30065298
-
Pancreatic tumor cell metastasis is restricted by MT1-MMP binding protein MTCBP-1.J Cell Biol. 2019 Jan 7;218(1):317-332. doi: 10.1083/jcb.201802032. Epub 2018 Nov 28. J Cell Biol. 2019. PMID: 30487181 Free PMC article.
-
Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling.Lab Invest. 2011 Mar;91(3):426-38. doi: 10.1038/labinvest.2010.201. Epub 2011 Jan 31. Lab Invest. 2011. Retraction in: Lab Invest. 2012 Dec;92(12):1801. doi: 10.1038/labinvest.2012.138. PMID: 21283078 Free PMC article. Retracted.
-
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia.J Cell Sci. 2009 Sep 1;122(Pt 17):3015-24. doi: 10.1242/jcs.034561. J Cell Sci. 2009. PMID: 19692588 Review.
-
Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion.Annu Rev Cell Dev Biol. 2016 Oct 6;32:555-576. doi: 10.1146/annurev-cellbio-111315-125227. Epub 2016 Aug 8. Annu Rev Cell Dev Biol. 2016. PMID: 27501444 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

