Obscurin deficiency leads to compensated dilated cardiomyopathy and increased arrhythmias
- PMID: 40366302
- PMCID: PMC12077377
- DOI: 10.1085/jgp.202413696
Obscurin deficiency leads to compensated dilated cardiomyopathy and increased arrhythmias
Abstract
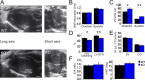
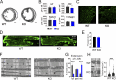
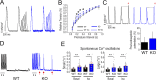
Obscurin is a large muscle protein whose multiple functions include providing mechanical strength to the M-band and linking the sarcomere to the sarcoplasmic reticulum. Mutations in obscurin are linked to various forms of muscle diseases. This study compares cardiac function in a murine model of obscurin deletion (KO) with wild-type (WT) in vivo and ex vivo. Echocardiography showed that KO hearts had larger (+20%) end-diastolic and end-systolic volumes, reduced fractional shortening, and impaired ejection fraction, consistent with dilated cardiomyopathy. However, stroke volume and cardiac output were preserved due to increased end-diastolic volume. Morphological analyses revealed reduced sarcoplasmic reticulum volume, with preserved T-tubule network. While myofilament function was preserved in isolated myofibrils and skinned trabeculae, experiments in intact trabeculae revealed that Obscn KO hearts compared with WT displayed (1) reduced active tension at high frequencies and during resting-state contractions, (2) impaired positive inotropic and lusitropic response to β-adrenergic stimulation (isoproterenol 0.1 μM), and (3) faster mechanical restitution, suggesting reduced sarcoplasmic reticulum refractoriness. Intracellular [Ca2+]i measurements showed reduced peak systolic and increased diastolic levels in KO versus WT cardiomyocytes. Western blot experiments revealed lower SERCA and phospholamban (PLB) expression and reduced PLB phosphorylation in KO mice. While action potential parameters and conduction velocity were unchanged, β-adrenergic stimulation induced more frequent spontaneous Ca2+ waves and increased arrhythmia susceptibility in KO compared with WT. Taken together, these findings suggest that obscurin deletion, in adult mice, is linked to compensated dilated cardiomyopathy, altered E-C coupling, impaired response to inotropic agents, and increased propensity to arrhythmias.
© 2025 Pioner et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures




References
-
- Ackermann, M.A., Hu L.Y., Bowman A.L., Bloch R.J., and Kontrogianni-Konstantopoulos A.. 2009. Obscurin interacts with a novel isoform of MyBP-C slow at the periphery of the sarcomeric M-band and regulates thick filament assembly. Mol. Biol. Cell. 20:2963–2978. 10.1091/mbc.e08-12-1251 - DOI - PMC - PubMed
-
- Armani, A., Galli S., Giacomello E., Bagnato P., Barone V., Rossi D., and Sorrentino V.. 2006. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp. Cell Res. 312:3546–3558. 10.1016/j.yexcr.2006.07.027 - DOI - PubMed
-
- Bang, M.L., Centner T., Fornoff F., Geach A.J., Gotthardt M., McNabb M., Witt C.C., Labeit D., Gregorio C.C., Granzier H., and Labeit S.. 2001. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 89:1065–1072. 10.1161/hh2301.100981 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 1.4 CUP B63C22000610006/National Recovery and Resilience Plan
- NNF22OC0079368/Novo Nordisk Fonden
- AUFF-E-2022-7-9/Aarhus Universitets Forskningsfond
- R01 HL152251/HL/NHLBI NIH HHS/United States
- 1.5 CUP B63C22000680007/National Recovery and Resilience Plan
- #3165-00028B/Independent Research Fund Denmark
- Next Generation European Union
- R396-2022-189/Lundbeck Foundation
- HL152251/NH/NIH HHS/United States
- Center for Gene Therapy and Drugs Based on RNA Technology
- HL128457/NH/NIH HHS/United States
- Tuscan Health Ecosystem
- R01 HL128457/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

