Blood-based biomarkers in soft tissue sarcoma: Implications for immune checkpoint inhibitor therapy
- PMID: 40366309
- PMCID: PMC12280857
- DOI: 10.1002/ijc.35477
Blood-based biomarkers in soft tissue sarcoma: Implications for immune checkpoint inhibitor therapy
Abstract
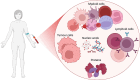
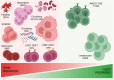
Soft tissue sarcoma (STS) is a rare and heterogeneous cancer, comprising approximately 1% of all adult cancers and 7%-15% of all childhood cancers. In the advanced stages, chemotherapy remains the standard-of-care, but efficacy is limited, with a response rate of 15%-30%, and responses are often short-lived, with median progression-free survival typically of 6 months. Moreover, patients with advanced or metastatic STS have a median overall survival of only 18-24 months. Immune checkpoint inhibitors (ICI) have revolutionized the treatment of various cancers including melanoma and non-small cell lung cancer (NSCLC). Emerging evidence from recent clinical trials indicates that certain STS subtypes may be amenable to immunotherapy. A critical challenge, however, is identifying biomarkers that can accurately predict and enable monitoring of ICI responses, to enable better patient selection and to improve outcomes. This narrative review highlights the current research gap in the treatment of STS patients with ICI therapy, particularly the absence of reliable blood-based biomarkers to predict ICI response. In this review, we examine current clinical trials investigating the efficacy of ICI therapy in patients with STS and summarise circulating immune-related prognostic biomarkers in STS, including haematological indices, peripheral blood mononuclear cells, circulating proteins and DNA, and evaluate their potential as predictive biomarkers for ICI therapy. We propose that these immune-associated molecules may serve as predictive biomarkers to differentiate and monitor ICI response, thus presenting opportunities for personalised treatment for patients with STS.
Keywords: circulating; clinical trials; immunotherapy; predictive; programmed cell death protein 1 (PD‐1).
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
All other authors declare that they have no competing interests.
Figures
References
-
- Katz D, Palmerini E, Pollack SM. More than 50 subtypes of soft tissue sarcoma: paving the path for histology‐driven treatments. Am Soc Clin Oncol Educ Book. 2018;38:925‐938. - PubMed
-
- Sarcomas . Soft Tissue: Statistics: American Society of Clinical Oncology. [https://www.cancer.net/cancer-types/sarcomas-soft-tissue/statistics]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

