Mitigation of Doxorubicin Cardiotoxicity With Synergistic miRNA Combinations Identified Using Combinatorial Genetics en masse (CombiGEM)
- PMID: 40366325
- PMCID: PMC12228136
- DOI: 10.1016/j.jaccao.2025.03.007
Mitigation of Doxorubicin Cardiotoxicity With Synergistic miRNA Combinations Identified Using Combinatorial Genetics en masse (CombiGEM)
Abstract
Background: Cardiomyocyte loss occurs in acute and chronic cardiac injury, including cardiotoxicity due to chemotherapeutics like doxorubicin, and contributes to heart failure development. There is a pressing need for cardiac-specific therapeutics that target cardiomyocyte loss, preventing chemotherapy complications without compromising chemotherapeutic efficacy.
Objectives: The authors employed massively parallel combinatorial genetic screening to find microRNA (miRNA) combinations that promote cardiomyocyte survival.
Methods: CombiGEM (combinatorial genetics en masse) screening in a cardiomyocyte cell line was followed by validation in the original cell type and screening in primary cardiomyocytes. The top combination was tested in mouse and developing zebrafish models of doxorubicin cardiotoxicity. RNA sequencing provided insight into possible mechanisms.
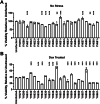
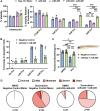
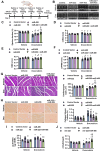
Results: Multiple miRNA combinations protected cardiomyocytes from doxorubicin in vitro. The most effective (miR-222+miR-455) appeared to act synergistically, and mitigated doxorubicin cardiotoxicity phenotypes in murine and zebrafish in vivo models. RNA sequencing revealed overlapping and synergistic regulation of relevant genes and biological processes in cardiomyocytes, including mitochondrial homeostasis, oxidative stress, muscle contraction, and others.
Conclusions: We identified miR-222 and miR-455 as a combination with potential therapeutic applications for cardioprotection. This study furthers our knowledge of the cardiac effects of miRNAs and their combinations and demonstrates the potential of CombiGEM for cardioprotective combinatorial therapeutic discovery.
Keywords: cardiac myocytes; cardiomyopathy; chemotherapy toxicity; cytoprotection; drug therapy; heart failure; innovation; preclinical study; systems biology.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by grants from the National Institutes of Health (to Dr Rosenzweig [R01HL135886, R35HL155318], Dr Platt [T32GM007226], Dr Chen [T32HL007208], Dr Asnani [K08HL145019], and Mr Bayer [F30HL162200]), the Department of Defense (to Dr Chen [W81XWH2110089]), the University of South Dakota Sanford School of Medicine (to Dr Chen [New Faculty Startup Fund]), the South Dakota Board of Regents (to Dr Chen [Governor’s Research Fund]), and the American Heart Association (to Dr Rosenzweig and Dr Lu [14CSA20500002 Collaborative Research Award], Dr Chen [855260 Career Development Award], and Dr Rosenzweig [026415-00001 Strategically Focused Research Network [SFRN] on Heart Failure, AHA SFRN [https://doi.org/10.58275/AHA.24SFRNPCN1284382.pc.gr.194135] and AHA MERIT Award]) and JSPS Grants-in-Aid (to Dr Uosaki [JP19KK0219 and JP23K24334] and Dr Tokuyama [JP24K10097]). Dr Higashikuni was supported by research fellowships from the Japan Heart Foundation and the Uehara Memorial Foundation, research grants from the Japan Foundation for Applied Enzymology, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Takeda Science Foundation, Tokyo Biochemical Research Foundation, Mitsui Sumitomo Insurance Welfare Foundation, Life Science Foundation of Japan, G-7 Scholarship Foundation, and Vehicle Racing Commemorative Foundation, and a JSPS KAKENHI Grant (JP19K23988). Dr Platt is now employed at Verve Therapeutics. Dr Kitchen is now employed at Novo Nordisk. Dr Lu is a co-founder of Senti Biosciences, Synlogic, Engine Biosciences, Tango Therapeutics, Corvium, BiomX, and Eligo Biosciences; holds financial interests in nest.bio, Ampliphi, IndieBio, Cognito Health, Quark Biosciences, Personal Genomics, Thryv, Lexent Bio, MitoLab, Vulcan, Serotiny, and Provectus Algae. Dr Uosaki has served as a scientific advisor for Molmir. Dr Asnani is a co-founder of and has served on the Board of Directors for Corventum, Inc. Dr Rosenzweig has served as a scientific co-founder and owns equity in Thryv Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Sawicki K.T., Sala V., Prever L., Hirsch E., Ardehali H., Ghigo A. Preventing and treating anthracycline cardiotoxicity: new insights. Annu Rev Pharmacol Toxicol. 2021;61:309–332. - PubMed
-
- Felker G.M., Thompson R.E., Hare J.M., et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

