Blood Pressure Lowering and Risk of Cancer: Individual Participant-Level Data Meta-Analysis and Mendelian Randomization Studies
- PMID: 40366326
- PMCID: PMC7618006
- DOI: 10.1016/j.jaccao.2025.03.005
Blood Pressure Lowering and Risk of Cancer: Individual Participant-Level Data Meta-Analysis and Mendelian Randomization Studies
Abstract
Background: Pharmacologic blood pressure (BP) lowering is typically a lifelong treatment, and both clinicians and patients may have concerns about the long-term use of antihypertensive agents and the risk for cancer. However, evidence from randomized controlled trials (RCTs) regarding the effect of long-term pharmacologic BP lowering on the risk for new-onset cancer is limited, with most knowledge derived from observational studies.
Objectives: The aim of this study was to assess whether long-term BP lowering affects the risk for new-onset cancer, cause-specific cancer death, and selected site-specific cancers.
Methods: Individual-level data from 42 RCTs were pooled using a one-stage individual participant data meta-analysis. The primary outcome was incident cancer of all types, and secondary outcomes were cause-specific cancer death and selected site-specific cancers. Prespecified subgroup analyses were conducted to assess the heterogeneity of the BP-lowering effect by baseline variables and over follow-up time. Cox proportional hazards regression, stratified by trial, was used for the statistical analysis. For site-specific cancers, analyses were complemented with Mendelian randomization, using naturally randomized genetic variants associated with BP lowering to mimic the design of a long-term RCT.
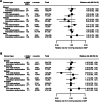
Results: Data from 314,016 randomly allocated participants without known cancer at baseline were analyzed. Over a median follow-up of 4 years (Q1-Q3: 3-5 years), 17,954 participants (5.7%) developed cancer, and 4,878 (1.5%) died of cancer. In the individual participant data meta-analysis, no associations were found between reductions in systolic or diastolic BP and cancer risk (HR per 5 mm Hg reduction in systolic BP: 1.03 [95% CI: 0.99-1.06]; HR per 3 mm Hg reduction in diastolic BP: 1.03 [95% CI: 0.98-1.07]). No changes in relative risk for incident cancer were observed over follow-up time, nor was there evidence of heterogeneity in treatment effects across baseline subgroups. No effect on cause-specific cancer death was found. For site-specific cancers, no evidence of an effect was observed, except a possible link with lung cancer risk (HR for systolic BP reduction: 1.17; 99.5% CI: 1.02-1.32). Mendelian randomization studies showed no association between systolic or diastolic BP reduction and site-specific cancers, including overall lung cancer and its subtypes.
Conclusions: Randomized data analysis provided no evidence to indicate that pharmacologic BP lowering has a substantial impact, either increasing or decreasing, on the risk for incident cancer, cause-specific cancer death, or selected site-specific cancers.
Keywords: epidemiology; genetics; hypertension; ischemic disease; lifestyle risk factors; lung cancer; prevention; renal cell cancer.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Funding Support and Author Disclosures This research was funded by the British Heart Foundation (PG/18/65/33872 and FS/IPBSRF/22/27060). Dr Rahimi has received grants outside the submitted work from the British Heart Foundation, the Horizon Europe AI4HF consortium (grant R79992/CN001), the Novo Nordisk Oxford Big Data Partnership, the University of Oxford, and UK Research and Innovation’s Global Challenge Research Fund (grant ES/P011055/1); has received consulting fees from Medtronic CRDN; has received honoraria or fees from Heart, PLoS Medicine, AstraZeneca MEA Region, Medscape, and WebMD Medscape UK; and is the editor-in-chief of Heart. Dr Canoy has received support from the UK Research and Innovation Medical Research Council (UKRI MRC) (MR/Y010825/1), the Dunhil Medical Trust (ARVHF2402/7), and the National Institute for Health and Care Research (NIHR) (NIR203982) outside of the submitted work; the views expressed are not necessarily those of these funders; he has also received an honorarium as Specialty Chief Editor of Frontiers in Cardiovascular Medicine (Cardiovascular Epidemiology and Prevention). Mrs Bidel has received a PhD fellowship from the British Heart Foundation (FS/PhD/25/29632). Dr Nazarzadeh is supported by a research fellowship from the British Heart Foundation (grant FS/IPBSRF/22/27060); has received reimbursement and honoraria from AstraZeneca, Nemysis, and Albus Health outside the submitted work; and is the statistical adviser for Heart. Dr Woodward has received personal fees from Amgen, Kyowa Kirin, and Freeline, outside the submitted work. Dr Sundström has ownership in companies providing services to Itrim, Amgen, Janssen, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Bayer, Pfizer, and AstraZeneca, outside the submitted work. Drs Rahimi and Canoy received grants from the British Heart Foundation during the conduct of the study. Dr Kjeldsen has received lecture honoraria from Emcure, Getz, Glenmark, J.B. Pharma, Merck, Vector-Intas, and Zydus; and has received study committee honoraria from Takeda. Dr Chalmers has received grants from the National Health and Medical Research Council of Australia, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Figures






References
-
- Piccirillo J.F., Tierney R.M., Costas I., Grove L., Spitznagel E.L., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. - PubMed
-
- Hidayat K., Du X., Zou S.Y., Shi B.M. Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 2017;35(7):1333–1344. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

