A Novel B7-H4xCD3 Bispecific T-cell Engager (PF-07260437) Synergizes with Breast Cancer Standard of Care and Immune Checkpoint Therapies
- PMID: 40366350
- PMCID: PMC12214883
- DOI: 10.1158/1535-7163.MCT-24-0379
A Novel B7-H4xCD3 Bispecific T-cell Engager (PF-07260437) Synergizes with Breast Cancer Standard of Care and Immune Checkpoint Therapies
Abstract
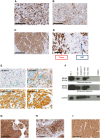
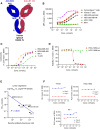
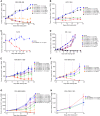
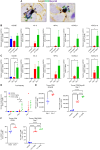
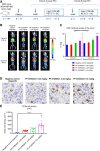
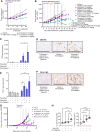
Immune checkpoint inhibitors have shown limited success in breast cancer, the most common and deadly cancer in women worldwide. Novel immune therapies, such as CD3-engaging bispecific antibodies, have shown clinical promise in hematologic malignancies. However, developing CD3 bispecifics for solid tumors has been challenging due to the difficulty in identifying tumor-specific antigens. B7-H4 is proposed as an attractive tumor-associated antigen for breast cancer therapeutics with comprehensive coverage regardless of breast cancer molecular subtype. We designed a B7-H4-targeting CD3 bispecific molecule, PF-07260437, and demonstrated B7-H4-dependent pharmacology in vitro by directing cytotoxic T-cell killing to breast cancer cell lines. Treatment of cell line- and patient-derived xenograft in vivo models of human breast cancer with PF-07260437 induced substantial tumoricidal activity, often resulting in complete responses. Mechanistically, PF-07260437 increased T-cell number and activation, leading to efficient tumor killing. Additionally, combining PF-07260437 with standard of care (palbociclib plus fulvestrant) and a checkpoint inhibitor (anti-PD-1) showed combinatorial benefits in an immune-competent in vivo model. Clinically relevant noninvasive PET/CT imaging with a CD8-targeting tracer demonstrated PF-07260437-mediated increases in intratumoral CD8 T cells, highlighting the utility of CD8-PET technology to potentially assess biomarker changes in the clinic. Finally, the manageable toxicity profile of PF-07260437 was highlighted in an exploratory toxicology study in cynomolgus monkeys. These data support the clinical testing of PF-07260437 for treating B7-H4-expressing solid tumors, including breast cancer.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
L. Wu reports personal fees from Pfizer during the conduct of the study and has a patent for WO2022013775A1 pending. M. Nocula-Lugowska reports other support from Pfizer, Inc. during the conduct of the study and has a patent for WO_2022_013775_A1 pending. E. Rosfjord reports employment with AstraZeneca and ownership of Pfizer and AstraZeneca stock. D. Mathur reports a patent for US11434292 (B2) issued. K. Maresca reports personal fees from Pfizer, Inc. outside the submitted work. A. Giddabasappa reports employment with Pfizer. C. Lees reports other support from Pfizer outside the submitted work and employment with Pfizer and ownership of Pfizer shares. A.T. Hooper reports other support and personal fees from Pfizer, Inc. during the conduct of the study as well as personal fees from Regeneron Pharmaceuticals outside the submitted work and has a patent for WO2022/013775 A1 pending. No disclosures were reported by the other authors.
Figures

References
-
- WHO . Breast cancer. Cancer 2020. Available from:https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
-
- Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA Cancer J Clin 2014;64:186–94. - PubMed
-
- Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 2015;35:85–95. - PubMed
-
- Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol 2003;171:4650–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

