Unraveling the role of stromal disruption in aggressive breast cancer etiology and outcomes
- PMID: 40366376
- PMCID: PMC12342738
- DOI: 10.1093/jnci/djaf070
Unraveling the role of stromal disruption in aggressive breast cancer etiology and outcomes
Abstract
Background: Aggressive (typically high-grade) breast cancers (BCs) remain major contributors to BC-related mortality globally. The tissue changes underpinning their etiology and outcomes, however, remain poorly characterized.
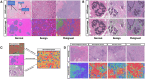
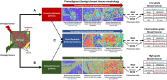
Methods: Spatially resolved machine-learning algorithms were used to characterize "stromal disruption" as a morphological metric of reduced/altered extracellular matrix and increased immune, inflammatory, and/or wound response-related processes in normal, benign breast disease (BBD), and invasive hematoxylin and eosin (H&E)-stained breast tissues. Associations of stromal disruption with BC etiologic factors were assessed among 4023 healthy breast tissue donors, its impact on BC incidence was assessed among 974 BBD patients in a nested case-control study, while its prognostic associations were assessed in 4 BC patient cohorts (n = 4223).
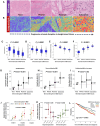
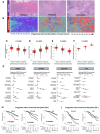
Results: Epidemiologic risk factors for aggressive BC, including younger age, multiparity, Black race, obesity, and family history, demonstrated strong associations with increasing stromal disruption in H&E sections prior to tumor development. Substantial stromal disruption in BBD H&E was associated with ∼4-fold increased risk of aggressive (high-grade) BC and ∼3 years shorter latency from BBD to BC diagnosis, independently of BBD histology. Across BC cohorts, stromal disruption in H&E was associated with aggressive (mostly high-grade) tumor phenotypes and with markedly poor prognosis among ER-positive patients, irrespective of histology. The immunobiology of stromal disruption reflected heightened innate (CD68+), adaptive (CD3+CD4+, CD3+CD8+), immunoregulatory (CD3+CD4+FOXP3+), immune escape (PD1+PDL1+), endothelial (CD31+), and myofibroblast (α-SMA+) marker expression.
Conclusion: Our findings highlight the active stromal role in aggressive BC etiology and outcomes, opening possibilities for readily identifying high-risk women across the BC continuum that may benefit from stroma-centric preventative or therapeutic strategies.
Published by Oxford University Press 2025.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. - PubMed
-
- Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324-3330. - PubMed
-
- Shinde SS, Forman MR, Kuerer HM, et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer. 2010;116:4933-4943. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

