A novel composite model for distinguishing benign and malignant pulmonary nodules
- PMID: 40366455
- PMCID: PMC12078401
- DOI: 10.1007/s10238-025-01672-5
A novel composite model for distinguishing benign and malignant pulmonary nodules
Abstract
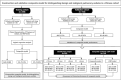
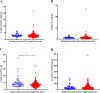
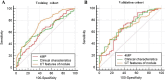
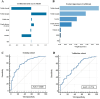
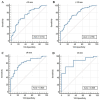
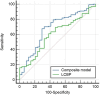
Previous studies have demonstrated that a four-protein marker panel (4MP), consisting of Pro-SFTPB, CA125, Cyfra21-1, and CEA could be used to identify benign and malignant lung nodules. This study aims to improve the 4MP's performance by combining clinical characteristics and low-dose chest computed tomography (LDCT) screening features. This study involved 380 patients with pulmonary nodules, diagnosing 91 benign and 289 early-stage lung cancer via postoperative histopathology. Serum levels of Pro-SFTPB, CA125, Cyfra21-1, and CEA were assessed using an immunofluorescence assay. Clinical features were selected using the LassoCV method. A new diagnostic model was developed using logistic regression, incorporating 4MP, clinical characteristics, and LDCT features. The model's diagnostic performance was compared to the lung cancer biomarker panel (LCBP) nodule risk model, and evaluated through sensitivity, specificity, and the AUC value. The AUC values for distinguishing between benign and malignant pulmonary nodules were 0.612 for the 4MP model. We screened out 7 factors of patient clinical information and CT features of nodules. The composite model (4MP + age + gender + BMI + family history of cancer + nodule size + nodule margin + nodule density) achieved an AUC of 0.808, especially for small nodules (AUC = 0.835 for nodules ≤ 6 mm). Furthermore, within the same validation cohort, the performance of the composite model (AUC = 0.680) surpassed that of the LCBP nodule risk model (AUC = 0.599). The novel composite model accurately diagnoses malignant pulmonary nodules, especially small ones, helping to stratify patients by lung cancer risk.
Keywords: Clinical characteristics; Composite model; Four-protein marker panel; Lung cancer; Pulmonary nodules.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: The authors declare no conflict of interest. Ethical approval: Ethics Statement: This work has been approved by the Medical Ethics Committee of Zhejiang Hospital (approval No. 2021-141 K). Consent to participate: All participants agreed to participate in this study, and signed the written informed consent to participate in this study. Consent to publish: All participants agreed to participate in this study and agreed to have their data published in a journal article.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

