Ravulizumab for generalized Myasthenia Gravis: a multicenter real-life experience
- PMID: 40366475
- PMCID: PMC12078421
- DOI: 10.1007/s00415-025-13127-8
Ravulizumab for generalized Myasthenia Gravis: a multicenter real-life experience
Abstract
Introduction: Ravulizumab, a monoclonal antibody targeting C5, was recently approved for the treatment of anti-AChR positive generalized myasthenia gravis (gMG) patients. The objective of this study is to present the Italian multicenter real-world experience evaluating the safety and efficacy of ravulizumab in gMG within the context of the Expanded Early Access Program (EAP).
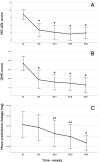
Methods: We conducted a retrospective study in 7 gMG referral centres in Italy. Demographic and clinical characteristics were recorded at baseline and during follow-up through clinical scale changes including Myasthenia Gravis-Activities of Daily Living (MG-ADL), Quantitative Myasthenia Gravis (QMG) and Myasthenia Gravis Composite (MGC). Frequency of minimal symptom expression (MSE) and changes in concomitant medications were also evaluated.
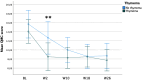
Results: Twenty-four gMG patients (10/24 females) aged between 24 and 82 years (Median 60.5, IQR 52.5-67.5), were included. Fifteen patients had undergone thymectomy, and 14 had a thymoma. Median follow-up duration was 26 weeks (range 10-74, IQR 26-42). MG-ADL and QMG scores showed a significant decrease with respect to baseline (p < 0.001). MSE was achieved by 37.5% patients at the last available follow-up. Tapering of prednisone daily dosage was possible in 76% of patients. Thymoma was significantly associated with QMG score reduction and the frequency of QMG responders at week 2 (p = 0.03). Three patients discontinued treatment. One patient experienced a myasthenic exacerbation and needed rescue therapy. Infectious adverse events were reported in 5/24 patients, and a Stevens-Johnson syndrome in one patient.
Conclusions: Real-world data confirm the effectiveness, safety, and prednisone-sparing effect of ravulizumab in patients with gMG, especially in those with thymoma.
Keywords: Complement inhibitor therapy; Myasthenia Gravis; Ravulizumab; Real life study.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: VDS received compensation for speaking from Alexion, UCB, Argenx and Alnylam; he is Sub-Investigator in clinical trials for Alexion, Alnylam, Argenx, Dianthus, and Sanofi. RI received speaker honoraria and consultancy fees from Alexion, Aera Therapeutics, Amgen, Argenx, Dianthus Therapeutics, Johnson&Johnson, Merck, and UCB. FH received speaker honoraria and consultancy fees from Argx, UCB, Alexion, Johnson Johnson MM received speaker honoraria and consultancy fees from Alexion, Argenx, UCB. CV has received honoraria for speakers, manuscript writing, educational events and support for attending meetings and travels from Alexion, ArgenX, UCB, and Sanofi. EMP received speaker honoraria and consultancy fees from Alexion, Argenx, UCB, Sanofi. DR has received honoraria for speakers, educational events and support for attending meeting and travels from Alexiox, UCB, Argenx and CSL Behring. SM received public speaking honoraria and travel grants from Alexion; MG received speaker honoraria and consultancy fees from Sanofi, Dianthus, Kedrion. LL received speaker honoraria and consultancy fees from Argx. GA received conference honoraria, advisory board and travel grants from Kedrion, Alnylam, Alexion, Argenx, Takeda; LF received public speaking honoraria and/or Advisory boards and travel grants from Alexion, Argenx, UCB, Dianthus. All the other authors reported no disclosures. Ethical approval: Written informed consent was obtained from the patient for the publication of anonymized data. We confirm that this work is in line with journal’s position on issues involved in ethical publication.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

