Autolysosomal Dysfunction in Obesity-induced Metabolic Inflammation and Related Disorders
- PMID: 40366502
- PMCID: PMC12078456
- DOI: 10.1007/s13679-025-00638-8
Autolysosomal Dysfunction in Obesity-induced Metabolic Inflammation and Related Disorders
Abstract
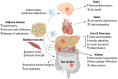
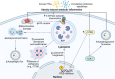
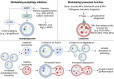
Purpose of review: Obesity is a global health crisis affecting individuals across all age groups, significantly increasing the risk of metabolic disorders such as type 2 diabetes (T2D), metabolic dysfunction-associated fatty liver disease (MAFLD), and cardiovascular diseases. The World Health Organization reported in 2022 that 2.5 billion adults were overweight, with 890 million classified as obese, emphasizing the urgent need for effective interventions. A critical aspect of obesity's pathophysiology is meta-inflammation-a chronic, systemic low-grade inflammatory state driven by excess adipose tissue, which disrupts metabolic homeostasis. This review examines the role of autolysosomal dysfunction in obesity-related metabolic disorders, exploring its impact across multiple metabolic organs and evaluating potential therapeutic strategies that target autophagy and lysosomal function.
Recent findings: Emerging research highlights the importance of autophagy in maintaining cellular homeostasis and metabolic balance. Obesity-induced lysosomal dysfunction impairs the autophagic degradation process, contributing to the accumulation of damaged organelles and toxic aggregates, exacerbating insulin resistance, lipotoxicity, and chronic inflammation. Studies have identified autophagic defects in key metabolic tissues, including adipose tissue, skeletal muscle, liver, pancreas, kidney, heart, and brain, linking autophagy dysregulation to the progression of metabolic diseases. Preclinical investigations suggest that pharmacological and nutritional interventions-such as AMPK activation, caloric restriction mimetics, and lysosomal-targeting compounds-can restore autophagic function and improve metabolic outcomes in obesity models. Autolysosomal dysfunction is a pivotal contributor to obesity-associated metabolic disorders , influencing systemic inflammation and metabolic dysfunction. Restoring autophagy and lysosomal function holds promise as a therapeutic strategy to mitigate obesity-driven pathologies. Future research should focus on translating these findings into clinical applications, optimizing targeted interventions to improve metabolic health and reduce obesity-associated complications.
Keywords: Autophagy function; Lysosomal acidification; Metabolic inflammation; Neurodegeneration; Neuroinflammation; Therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Compliance with Ethical Standards. Conflict of interests: The authors declare no competing interests. Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures
Similar articles
-
Palmitic acid-induced autolysosomal dysfunction and lipotoxicity in neuroinflammation and neurodegeneration.Neural Regen Res. 2025 Jul 5. doi: 10.4103/NRR.NRR-D-25-00432. Online ahead of print. Neural Regen Res. 2025. PMID: 40618261
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Interferon regulatory factor 3 beyond innate immunity: Regulation in obesity and metabolic disorders.Semin Immunol. 2025 Jun;78:101948. doi: 10.1016/j.smim.2025.101948. Epub 2025 Mar 28. Semin Immunol. 2025. PMID: 40156960 Review.
-
Mechanisms underlying the interplay between autophagy and the inflammasome in age-related diseases: Implications for exercise immunology.Ageing Res Rev. 2025 Aug;110:102821. doi: 10.1016/j.arr.2025.102821. Epub 2025 Jul 1. Ageing Res Rev. 2025. PMID: 40609647 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

