Targeting brain health in subjective cognitive decline: insights from a multidomain randomized controlled trial
- PMID: 40366507
- PMCID: PMC12078420
- DOI: 10.1007/s40520-025-03062-z
Targeting brain health in subjective cognitive decline: insights from a multidomain randomized controlled trial
Abstract
Background: Multidomain lifestyle interventions are a promising approach to prevent cognitive decline, but their effects in subjective cognitive decline (SCD) remain controversial. We investigated the effects of lifestyle interventions on cognition and brain integrity in these at-risk individuals.
Methods: One-hundred twenty-eight older adults with SCD were randomly assigned to either Active Control Intervention (ACI), i.e. health education; Partial Intervention (PI), i.e. tramiprosate supplementation (100 mg/die) and dietary advice; or Multilevel Intervention (MI), i.e. PI plus computerized cognitive training and physical exercise, for one year. Neuropsychological assessment and MRI were performed at baseline and at 1-year follow-up. Analyses of covariance were used to measure the effects of interventions on predefined outcomes.
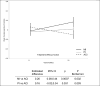
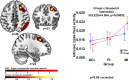
Results: The MI group significantly improved in attention-executive functioning (p = 0.003) compared to ACI (Cohen's d: 0.47, 95% CI 0.13-0.79). In addition, depressive symptoms (Cohen's d: - 0.48, 95% C.I. - 0.81 to - 0.14) and memory concerns (Cohen's d: - 0.77, 95% C.I. - 1.12 to - 0.41) decreased in the MI and PI respectively, relative to the ACI. The MI group also showed increased resting-state (i.e., intrinsic) brain activity in the right fronto-parietal executive network. No significant intervention effects on brain structural or vascular outcomes were found.
Conclusion: The study shows that a multidomain lifestyle intervention can enhance attention-executive function, ameliorate depressive symptoms and increase functional connectivity in SCD. These findings support the role of lifestyle interventions in public health strategies to mitigate cognitive decline risk.
Trial registration: The trial has been registered at the United States National Library of Medicine at the National Institutes of Health Registry of Clinical Trials under the code NCT04744922 on December 9th, 2017 ( https://www.
Clinicaltrials: gov/ct2/show/NCT03382353 ).
Keywords: Dementia prevention; Lifestyle; Multidomain interventions; Subjective cognitive decline; fMRI.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval and consent to participate: The study was performed in accordance with the guidelines of the Declaration of Helsinki. The study protocol was approved by the local ethics committees (Ethic Committee of the IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli and Ethic Committee of the San Raffaele hospital). Written informed consent was collected for all of the participants before screening evaluation. The principles of good clinical practice were observed in conducting the study.
Figures


References
-
- World Health Organization and Alzheimer’s Disease International. Dementia: a public health priority 2012:112.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

