Tobacco Smoking Rewires Cell Metabolism by Inducing GAPDH Succinylation to Promote Lung Cancer Progression
- PMID: 40366632
- PMCID: PMC12314509
- DOI: 10.1158/0008-5472.CAN-24-3525
Tobacco Smoking Rewires Cell Metabolism by Inducing GAPDH Succinylation to Promote Lung Cancer Progression
Abstract
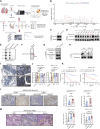
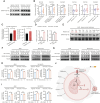
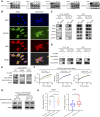
Patient behavior and physiology can directly affect cancer metabolism. Smoking is the leading risk factor for non-small cell lung cancer (NSCLC). In this study, we identified that smoking modulates lung cancer cell metabolism through altered protein post-translational modification. Proteomic analyses identified elevated K251 succinylation (K251-Su) of GAPDH, a key enzyme in glycolysis, in NSCLC samples, and GAPDH K251-Su was significantly higher in patients who smoke compared with nonsmokers. Exposure of lung cancer cells to cigarette smoke extract led to increased uptake of glutamine and enhanced GAPDH K251-Su. Glutamine uptake by cancer cells in hypoxic and nutrient-deficient microenvironments provided succinyl-CoA donors for GAPDH succinylation at K251, which was catalyzed by acyltransferase p300. K251-Su increased GAPDH stability by suppressing TRIM4-mediated K254 ubiquitination. GAPDH K251-Su enhanced glycolysis and glutamine reductive carboxylation to meet the demands for cell growth and to support survival in hypoxic and nutrient-depleted conditions, promoting tumor growth and metastasis. These findings indicate that tobacco smoking mediates metabolic reprogramming of cancer cells through succinylation of GAPDH to drive NSCLC progression.
Significance: Smoking-induced GAPDH succinylation coordinates glycolysis and glutamine metabolism and supports lung cancer cell survival in stressful microenvironments to promote tumor progression, highlighting quitting smoking as a potential strategy to target cancer metabolism.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
No disclosures were reported.
Figures



References
-
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol 2023;20:624–39. - PubMed
-
- Caini S, Del Riccio M, Vettori V, Scotti V, Martinoli C, Raimondi S, et al. Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: a systematic review and meta-analysis. J Thorac Oncol 2022;17:623–36. - PubMed
-
- Liu Y, Lu L, Yang H, Wu X, Luo X, Shen J, et al. Dysregulation of immunity by cigarette smoking promotes inflammation and cancer: a review. Environ Pollut 2023;339:1–17. - PubMed
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov 2022;12:31–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

