Spironolactone vs Amiloride for Resistant Hypertension: A Randomized Clinical Trial
- PMID: 40366680
- PMCID: PMC12079568
- DOI: 10.1001/jama.2025.5129
Spironolactone vs Amiloride for Resistant Hypertension: A Randomized Clinical Trial
Abstract
Importance: Amiloride has been proposed as an alternative to spironolactone for treating resistant hypertension. However, no randomized clinical trials have compared the efficacy of spironolactone and amiloride in patients with resistant hypertension.
Objective: To determine whether amiloride is noninferior to spironolactone in reducing home-measured systolic blood pressure (SBP) in patients with resistant hypertension.
Design, setting, and participants: Prospective, open-label, blinded end-point randomized clinical trial conducted at 14 sites in South Korea. From November 16, 2020, to February 29, 2024, 118 patients with home SBP of 130 mm Hg or greater after a 4-week run-in period with a fixed-dose triple medication combination (angiotensin receptor blocker, calcium channel blocker, and thiazide) were enrolled.
Intervention: Patients were randomized in a 1:1 ratio to receive 12.5 mg/d of spironolactone (n = 60) or 5 mg/d of amiloride (n = 58). If home SBP remained 130 mm Hg or greater and serum potassium was less than 5.0 mmol/L after 4 weeks, dosages were increased to 25 mg/d and 10 mg/d, respectively.
Main outcomes and measures: The primary end point was the between-group difference in home SBP change at week 12, with a noninferiority margin of -4.4 mm Hg for the lower bound of the confidence interval. Secondary end points included achievement rates of home- and office-measured SBP of less than 130 mm Hg.
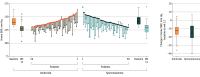
Results: The median age of the study population was 55 years, with 70% male. There were no differences between groups in demographic characteristics other than use of α-blockers (8.6% in the amiloride group and 0% in the spironolactone group). The mean baseline home SBPs were 141.5 (SD, 7.9) mm Hg and 142.3 (SD, 8.5) mm Hg in the amiloride and spironolactone groups, respectively. At week 12, mean home SBP measurements were changed from baseline by -13.6 (SD, 8.6) mm Hg and -14.7 (SD, 11.0) mm Hg in the amiloride and spironolactone groups, respectively (between-group difference in change, -0.68 mm Hg; 90% CI, -3.50 to 2.14 mm Hg), with amiloride demonstrating noninferiority to spironolactone. Home-measured achievement rates of SBP less than 130 mm Hg in the amiloride and spironolactone groups were 66.1% and 55.2%, respectively, and office-measured achievement rates of SBP less than 130 mm Hg were 57.1% and 60.3%, respectively, with no difference between the 2 groups. One case of hyperkalemia-related discontinuation occurred in the amiloride group, with no cases of gynecomastia in either group.
Conclusions and relevance: Amiloride was noninferior to spironolactone in lowering home SBP, suggesting that it could be an effective alternative for treatment of resistant hypertension.
Trial registration: ClinicalTrials.gov Identifier: NCT04331691.
Conflict of interest statement
Figures

Comment in
-
New Support for Old Medications in Resistant Hypertension.JAMA. 2025 Jun 17;333(23):2058-2060. doi: 10.1001/jama.2025.4321. JAMA. 2025. PMID: 40366665 No abstract available.
References
-
- Carey RM, Calhoun DA, Bakris GL, et al. ; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Genomic and Precision Medicine, Council on Peripheral Vascular Disease, Council on Quality of Care and Outcomes Research, and Stroke Council . Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53-e90. doi: 10.1161/HYP.0000000000000084 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

