Reversal of morphine-induced respiratory depression with the µ1-opioid receptor antagonist naloxonazine engenders excitation and instability of breathing
- PMID: 40366705
- PMCID: PMC12213126
- DOI: 10.1152/ajplung.00045.2025
Reversal of morphine-induced respiratory depression with the µ1-opioid receptor antagonist naloxonazine engenders excitation and instability of breathing
Abstract
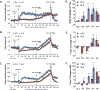
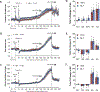
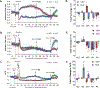
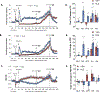
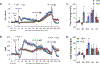
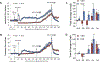
The administration of opioid receptor antagonists is believed to overcome ventilatory depressant effects of opioids. Here we show that many ventilatory depressant effects of morphine are converted to excitatory responses after µ1-opioid receptor blockade, and that these responses are accompanied by ventilatory instability. In this study, we report 1) ventilatory responses elicited by morphine (10 mg/kg, iv) and 2) ventilatory responses elicited by a subsequent hypoxic-hypercapnic (HH) gas challenge and return to room air in male Sprague Dawley rats pretreated with 1) vehicle, 2) the centrally acting selective µ1-opioid receptor antagonist, naloxonazine (1.5 mg/kg, iv), or 3) the centrally acting (delta 1,2) δ1,2-opioid receptor antagonist, naltrindole (1.5 mg/kg, iv). The morphine-induced decreases in frequency of breathing, peak inspiratory flow, peak expiratory flow, expiratory flow at 50% expired TV, inspiratory drive, and expiratory drive in vehicle-treated rats were converted to profound increases in naloxonazine-treated rats. Additionally, the adverse effects of morphine on expiratory delay and apneic pause were augmented in naloxonazine-treated rats, and administration of morphine increased ventilatory instability (i.e., noneupneic breathing index) in naloxonazine-treated rats, which was not due to increases in ventilatory drive. Subsequent exposure to a HH gas challenge elicited qualitatively similar responses in both groups, whereas the responses upon return to room air (e.g., frequency of breathing, inspiratory time, expiratory time, end expiratory pause, relaxation time, expiratory delay, and noneupneic breathing index) were substantially different in naloxonazine-treated versus vehicle-treated rats. The above mentioned effects of morphine were only marginally affected in naltrindole-treated rats. These novel data highlight the complicated effects that µ1-opioid receptor antagonism exerts on the ventilatory effects of morphine.NEW & NOTEWORTHY This study shows that the systemic injection of morphine elicits a pronounced overshoot in ventilation in freely-moving Sprague Dawley rats pretreated with the centrally-acting selective µ1-opioid receptor antagonist, naloxonazine, but not with the centrally-acting δ1,2-opioid receptor antagonist, naltrindole. This suggests that morphine can recruit a non-µ1-opioid receptor system that promotes breathing.
Keywords: male Sprague Dawley rats; morphine; naloxonazine; ventilatory depression; ventilatory instability.
Figures



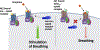
References
-
- Olofsen E, van Dorp E, Teppema L, Aarts L, Smith TW, Dahan A, Sarton E. Naloxone reversal of morphine- and morphine-6-glucuronide-induced respiratory depression in healthy volunteers: a mechanism-based pharmacokinetic-pharmacodynamic modeling study. Anesthesiology 112: 1417–1427, 2010. doi: 10.1097/ALN.0b013e3181d5e29d. - DOI - PubMed
-
- Henderson F, May WJ, Gruber RB, Discala JF, Puskovic V, Young AP, Baby SM, Lewis SJ. Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats. Respir Physiol Neurobiol 191: 95–105, 2014. doi: 10.1016/j.resp.2013.11.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

