Targeted transarterial embolization of the femoral head: development of a minimally invasive approach to model Legg-Calvé-Perthes disease in piglets
- PMID: 40367088
- PMCID: PMC12077684
- DOI: 10.1371/journal.pone.0323360
Targeted transarterial embolization of the femoral head: development of a minimally invasive approach to model Legg-Calvé-Perthes disease in piglets
Abstract
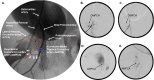
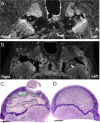
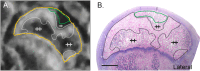
Clinical management of children with Legg-Calvé-Perthes Disease (LCPD) is hampered by incomplete understanding of how the extent of ischemic injury and the duration and quality of subsequent repair determine patient outcome. The traditional piglet model of LCPD is limited to capturing global femoral head ischemia; thus, a new model is needed in which the extent of ischemia can be varied to replicate the spectrum of disease seen in children. In this exploratory study, we used an iterative approach to test and refine methods to bilaterally occlude vessels supplying the femoral heads in n = 8 young piglets under angiographic control. The deep and/or acetabular medial femoral circumflex arteries (DMFCA and AMFCA) were identified and embolized using either embolic particles or liquid embolic agents. The extent of ischemia was assessed immediately post-embolization (4 piglets) and/or 7 days following embolization (7 piglets) using contrast-enhanced magnetic resonance imaging (CE-MRI). After the final CE-MRI, piglets were euthanized, and their femora were harvested for histologic evaluation. Embolization of the DMFCA alone caused transient ischemia that largely resolved by 7 days with small regions of fibrovascular repair of ischemic injury remaining on histology. Embolization of both the DMFCA and AMFCA resulted in a greater degree of pathologic changes at 7 days post-operatively, but also with nearly complete restoration of femoral head perfusion. We found that combining injection of embolic particles with subsequent placement of an embolic micro-coil was the most effective approach to induce ischemic injury, which may be aided in larger piglets. While our findings should be interpreted cautiously due to the wide range in the age and size of animals investigated, they demonstrate that transarterial embolization of the vascular supply of the femoral head results in transient ischemia and histological changes consistent with partial ischemic injury. These results will inform further development of a minimally invasive piglet model of LCPD that offers a unique representation of the spectrum of pathophysiology of LCPD compared to the traditional model.
Copyright: © 2025 Novotny et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Jennifer C. Laine is a board member of the Pediatric Society of North America and an Executive Board member of the International Perthes Study Group. Susan A. Novotny is a member of the Steering Committee of the International Perthes Study Group. The remaining authors have declared that no competing interests exist.
Figures


Similar articles
-
Intravoxel incoherent motion (IVIM) detects femoral head ischemia in a piglet model of Legg-Calvé-Perthes disease.J Orthop Res. 2024 Apr;42(4):855-863. doi: 10.1002/jor.25733. Epub 2023 Dec 4. J Orthop Res. 2024. PMID: 37971281 Free PMC article.
-
Quantitative T2 and T1ρ mapping are sensitive to ischemic injury to the epiphyseal cartilage in an in vivo piglet model of Legg-Calvé-Perthes disease.Osteoarthritis Cartilage. 2022 Sep;30(9):1244-1253. doi: 10.1016/j.joca.2022.05.009. Epub 2022 May 26. Osteoarthritis Cartilage. 2022. PMID: 35644462 Free PMC article.
-
Detection of early metaphyseal changes in a piglet model of Legg-Calvé-Perthes disease using quantitative mapping of MRI relaxation times.J Orthop Res. 2024 Oct;42(10):2277-2286. doi: 10.1002/jor.25904. Epub 2024 May 26. J Orthop Res. 2024. PMID: 38796746 Free PMC article.
-
[Legg-Calvé-Perthes disease].Radiologie (Heidelb). 2023 Oct;63(10):736-744. doi: 10.1007/s00117-023-01182-z. Epub 2023 Jul 8. Radiologie (Heidelb). 2023. PMID: 37422572 Review. German.
-
Legg-Calvé-Perthes disease overview.Orphanet J Rare Dis. 2022 Mar 15;17(1):125. doi: 10.1186/s13023-022-02275-z. Orphanet J Rare Dis. 2022. PMID: 35292045 Free PMC article. Review.
References
-
- Kim HK. Legg-Calve-Perthes disease. J Am Acad Orthop Surg. 2010;18(11):676-86. - PubMed
-
- Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. part II: prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86-a(10):2121-34. - PubMed
-
- J Bone Joint Surg Am Larson AN, Sucato DJ, Herring JA, Adolfsen SE, Kelly DM, Martus JE, et al.. A prospective multicenter study of Legg-Calvé-Perthes disease: functional and radiographic outcomes of nonoperative treatment at a mean follow-up of twenty years. J Bone Joint Surg Am. 2012;94(7):584–92. doi: 10.2106/JBJS.J.01073 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

